Upper-extremity support OKG-14


 Finger brace
Finger brace Anatomic wrist splint
Anatomic wrist splint Breathable
Breathable Class I medical device
Class I medical device Double-sided
Double-sided Skin-friendly
Skin-friendly Universal size
Universal sizeHAND FINGERS SPLINT
Description
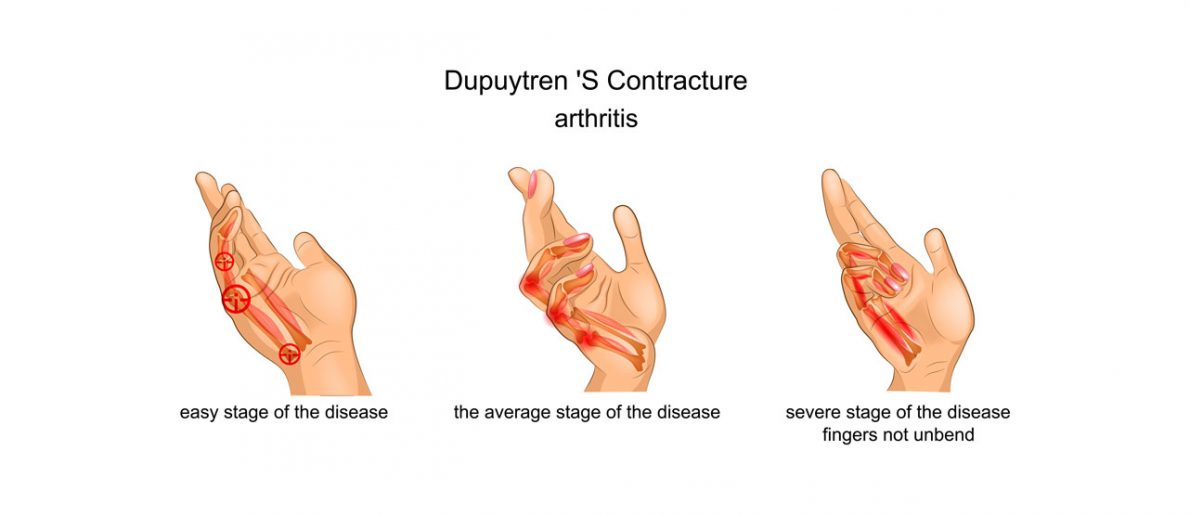
Dupuytren’s Contracture
Dupuytren’s Contracture is a hand deformity that usually develops over years. Can be hereditary. It is an abnormal thickening of the skin in the palm of the hand. This thickened area may develop into a hard lump or thick band and may result in muscles contractures. This condition maintains the fingers in bend position. The affected fingers can’t be straightened completely, which can complicate everyday activities such as wearing, car driving or cooking. Treatment for severe stage of Dupuytren’s contracture includes surgery. The surgeon makes a cut in the hand and takes out the thickened tissue.
Unfortunately, the condition is chronic and some people have contractures return so they may need surgery again. Due to this fact, it is recommended to use hand fingers splint such as OKG-14 which maintains the tissues stretching and prevents against the contracture.
Product’s description
Hand fingers brace OKG-14 is an excellent product for fingers, other than thumb, immobilization. The brace has unique design and offers II-III and IV-V fingers immobilization. It provides passive tissues redresion, improving elasticity and reducing contractures.
The aluminum splint is made of low profiled “Y” shaped aluminum which involves the DIP (distal interphalangeal), PIP (proximal interphalangeal) and MCP (metacarpophalangeal) and wrist joints of the hand.
Bendable aluminum splint offers safe immobilization and match the natural curve of the hand and fingers.
The aluminum splint is covered with innovative fabric AeroSpace II™ offering air flow and skin’s breathability.
What is more, hand fingers splint OKG-14 is equipped with adjustable straps which move along the hard splint to fit the shape of the wrist, palm, and fingers, offering comfort of use.
Our hand fingers splint OKG-14 has ‘FREE HANDS’ design and keeps the thumb free for improving daily activities.
Our hand fingers splint OKG-14 keeps the soft tissues stretched and improves their elasticity. It prevents against fingers contractures. The hand splint maintains fingers in neutral position, limits finger movement, promotes natural healing and prevents re-injury.
Our hand fingers splint OKG-14 prevents against the condition and also may be as a immobilization used after surgery.
Apart of Dupuytren’s contracture, the brace may be used for other conditions requiring fingers immobilization.
Purpose of use
– Dupuytren’s contracture
– Trigger Finger
– fingers sprain, bruise or dislocation
– hand arthritis
– hand tendonitis
– metacarpal fractures (boxer fracture).
– stroke
– ulnar claw
– boutonniere deformity
– swan neck deformity
Sizes
| Size | Wrist circumference | How to measure |
| Universal | min 11 – max 24 cm | 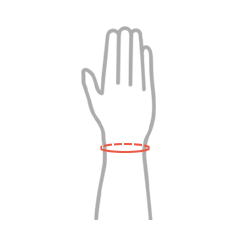 |
Fits for both hands.
Total length of the product: 30 cm.
Gallery
Technology
MATERIALS
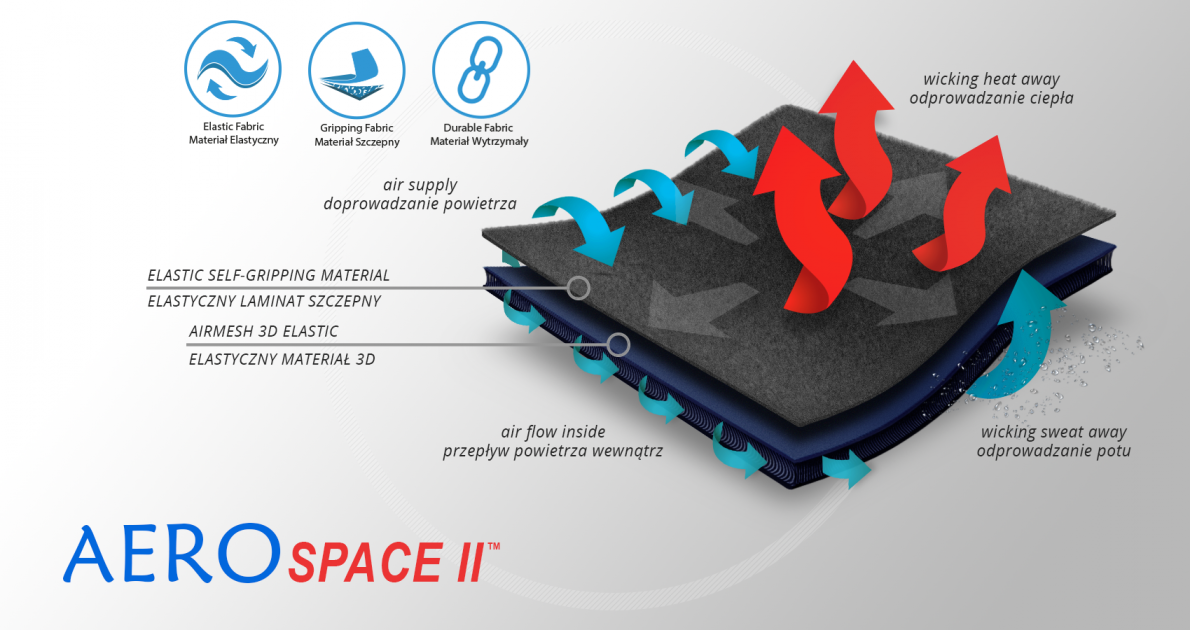
AeroSpace II™
AeroSpace II™ is an innovative new generation raw material. It is a distance elastic 3D knitted fabric consisting of two layers of facings and an interlacing that creates the appropriate thickness of the raw material and has relieving properties. The knitwear is made of the highest quality polyester yarn - guaranteeing high mechanical strength and spandex ensuring its proper flexibility. AeroSpace II™ has an external self-gripping layer what makes it easy to adjust each product to individual patient’s needs. This material is characterized by a very low weight, high flexibility and a very large openwork structure, allowing for very easy drainage of sweat from the body and bringing fresh air to the skin. Products made of this raw material are neutral to the secured joint, do not heat or cool it, but ensure its proper compression and fit and reduce muscle vibrations generated during physical exertion. Its thickness and 3D structure perfectly relieves the orthopedic splints, stays or other elements mounted on the product and guarantees velvety softness to the touch.

STIFFENINGS
Aluminum frame
Setting up
Downloads
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.