Upper limb support AM-SOB-04


 Upper limb brace
Upper limb brace Breathable
Breathable Class I medical device
Class I medical device Cotton
Cotton Latex-free
Latex-free Skin-friendly
Skin-friendlyARM SLING
Product description
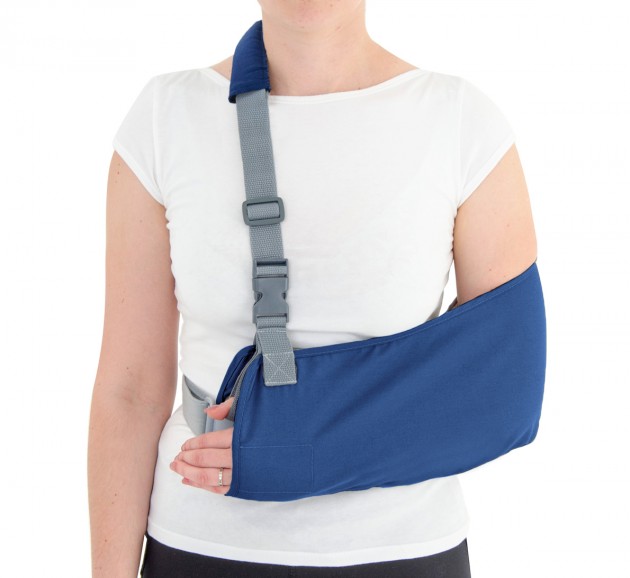
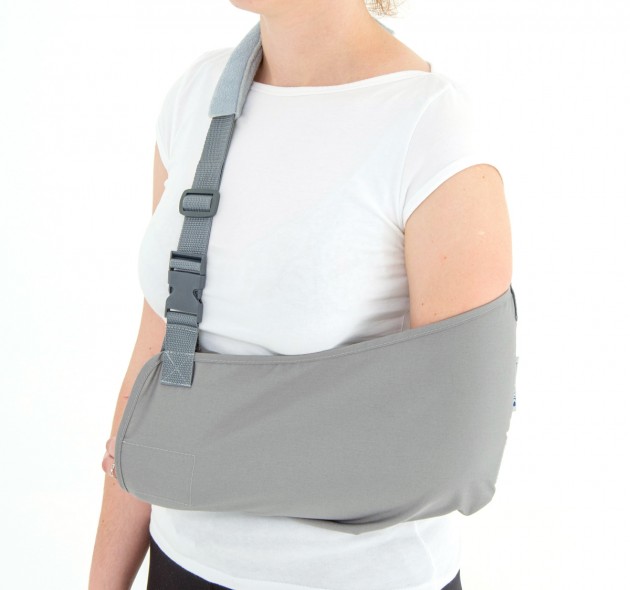
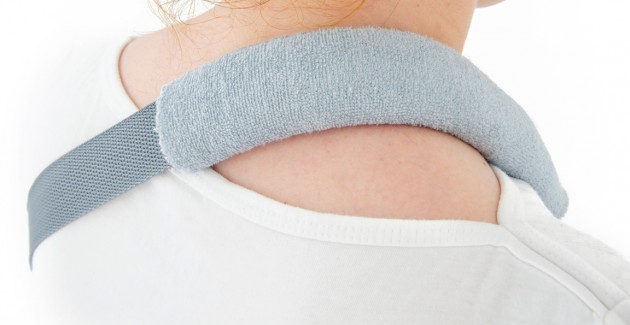
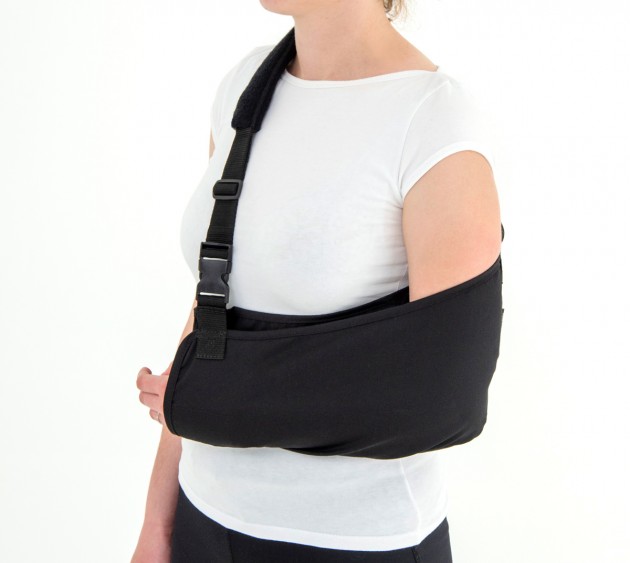
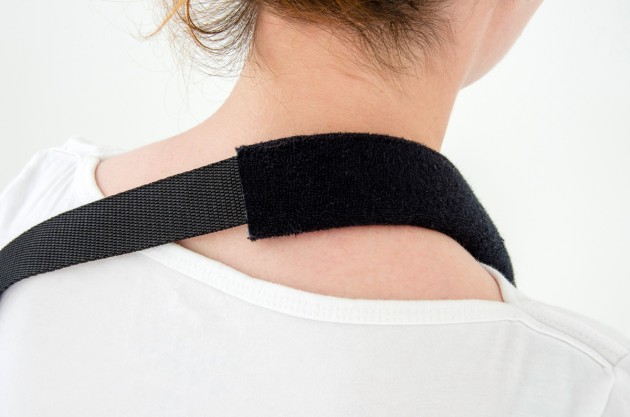
Orthosis AM-SOB-04 has been designed and constructed in such a way that its use caused the least problems to the patient that he can wear it on alone, and lightweight design facilitate its use on a daily basis.
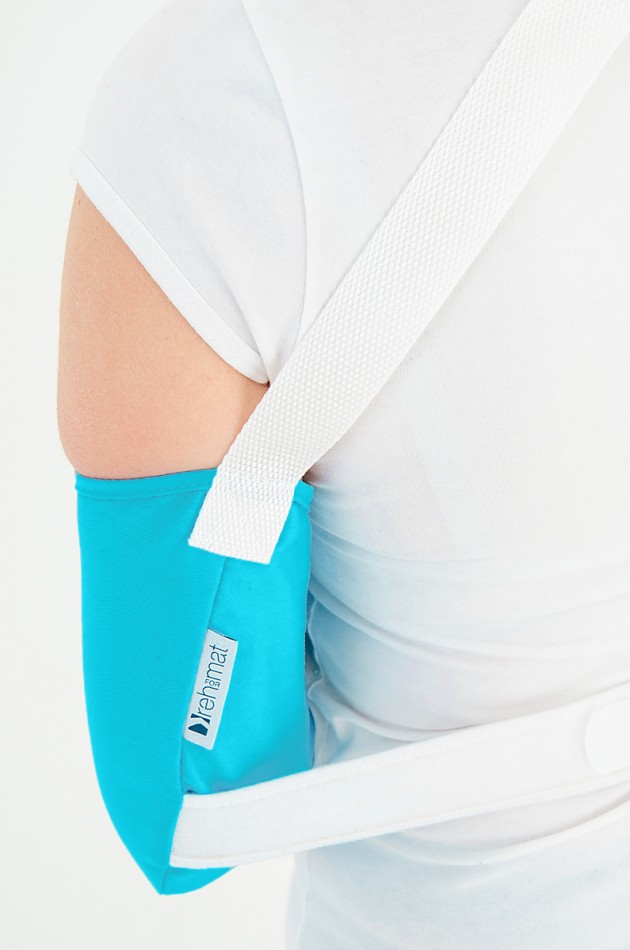
The orthese sets arm and forearm in proper position during the treatment of shoulder dysfunction. The arm can be set in neutral position or slightly approached.
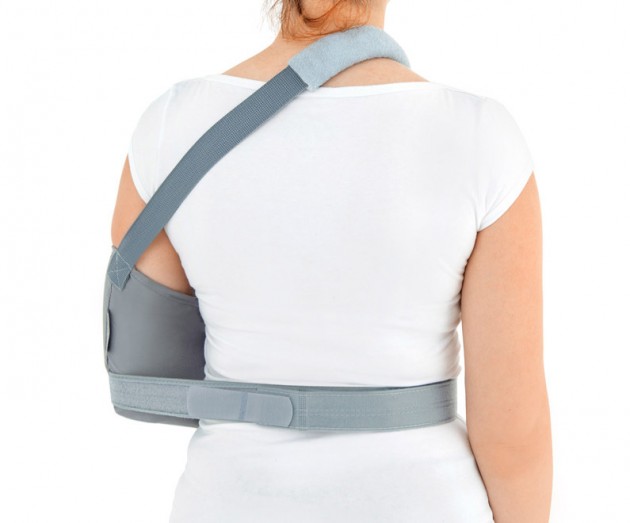
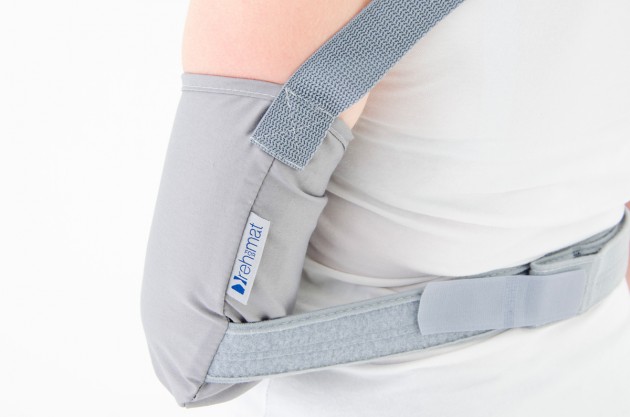
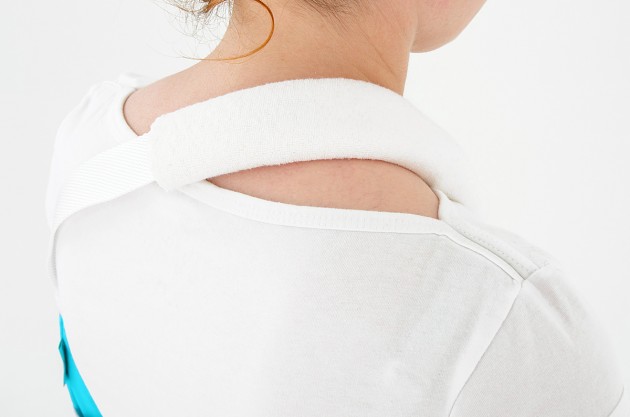
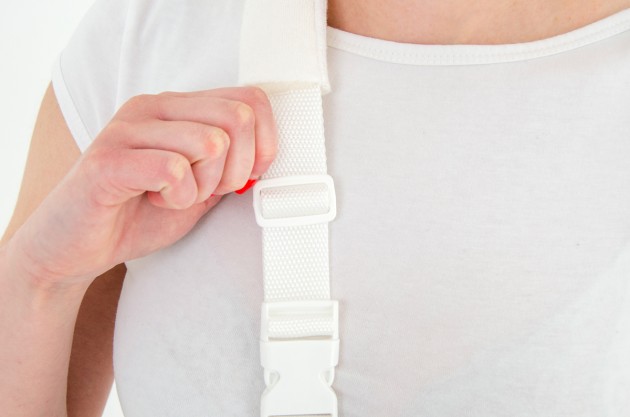
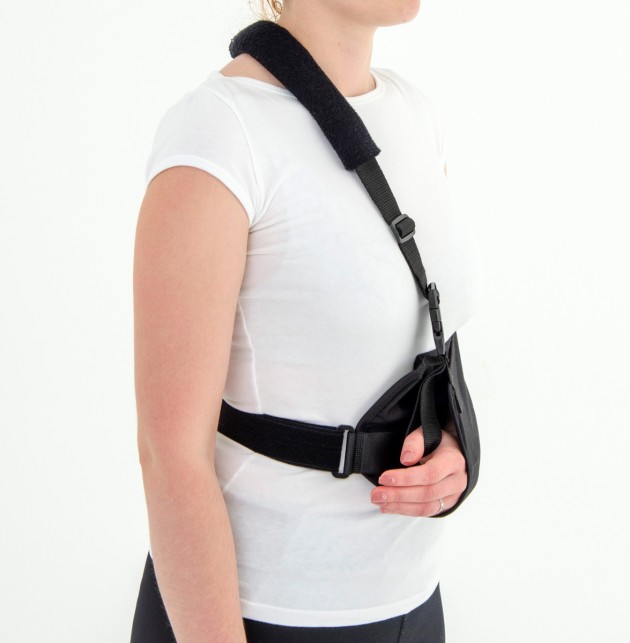
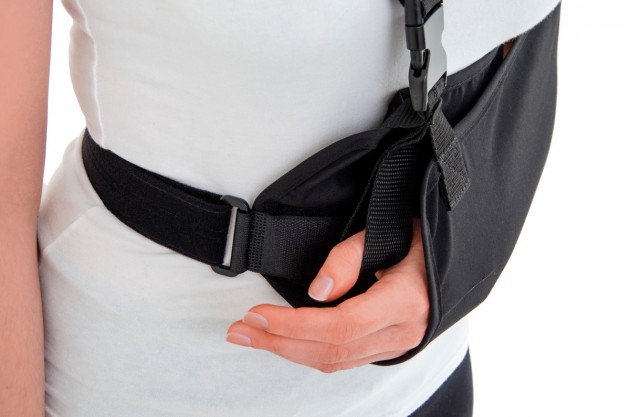
Each of the points of attachment of the orthosis can be independently adjusted for optimal fit in all the circumstances.
Due to the design of a product can be used on both the right or left arm.
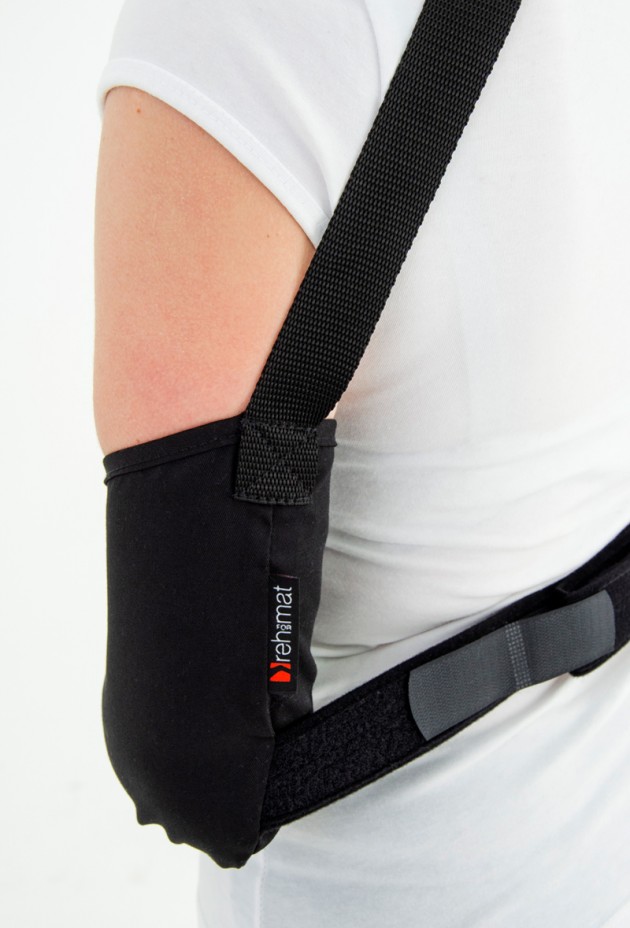
The product is equipped with universal shoulder strap and hand sling.
Available versions of sling AM-SOB-04:
The AM-SOB-04 arm sling is made UniCotton™.
Purpose of use
The shoulder stabilization should be used in following cases:
– strong pain in the shoulder joint (temporary protection)
– dislocation of the joint
– injury to the soft tissue of the shoulder and arm
– surgery of the shoulder or elbow (not requiring plaster dressing)
– in case of necessity to support or partially fix the upper limb (broken neck of humerus bone and plaster dressing)
– after removal of the plaster dressing, as stabilization
Available sizes
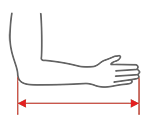
| Size | The length of the forearm and hand | How to measure |
| S | 30-36 cm (11,8″-14,2″) |
 |
| M | 36-42 cm (14,2″-16,5″) |
|
| L | 42-48 cm (16,5″-18,9″) |
|
| XL | 48-54 cm (18,9″-21,3″) |
|
| 2XL | 54-60 cm (21,3″-23,6″) |
|
| 3XL | 60-64 cm (23,6″-25,2″) |
| Size | Total height of the product | Total length of product |
| S | 18 cm (7,1″) | 33 cm (13″) |
| M | 20 cm (7,9″) | 39 cm (15,4″) |
| L | 22 cm (8,7″) | 46 cm (18,1″) |
| XL | 25 cm (9,8″) | 53 cm (20,9″) |
| 2XL | 26 cm (10,2″) | 59 cm (23,2″) |
| 3XL |
Fits for both forearms.
Technology
MATERIALS
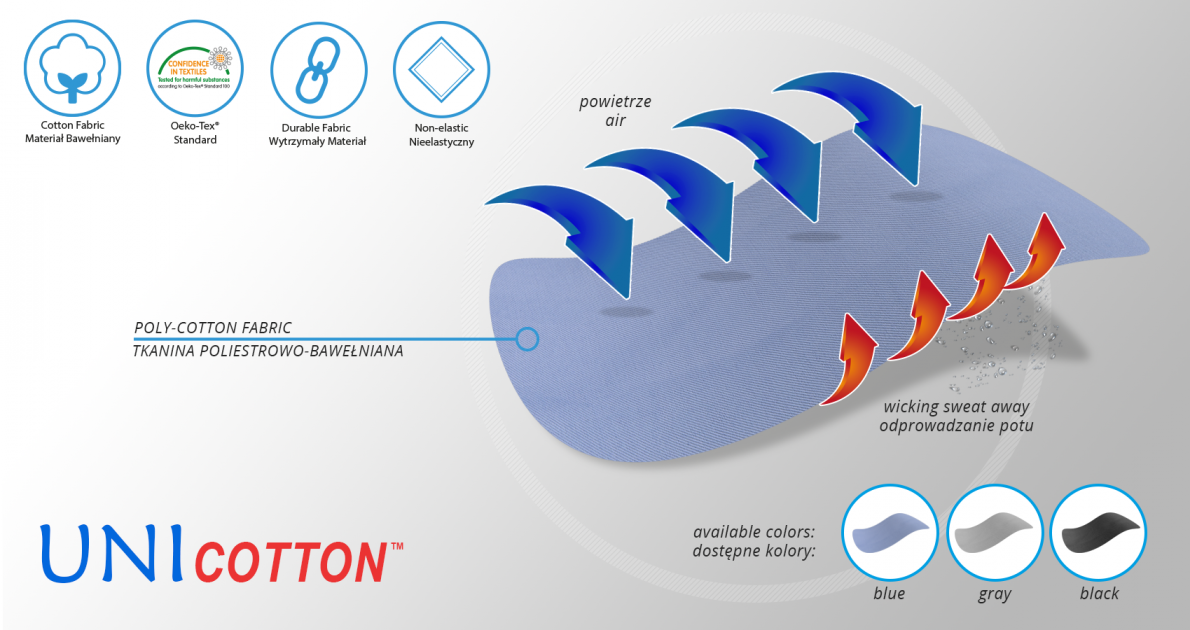
UniCotton™
UniCotton™ is a polyester-cotton fabric with versatile use. The fabric is available depending on the needs in different colours and weights, is characterized by high durability and very good performance parameters, it has an Oeko-Tex Standard 100 certificate, which confirms the fact that it is inert to the patient’s skin.
Setting up
Downloads
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.