AM-SX-02/CCA

 Ankle brace
Ankle brace Anatomic side supports
Anatomic side supports CCA
CCA Class I medical device
Class I medical device Double-sided
Double-sided ER
ER Innovative
InnovativeAM-SX-02/CCA – ANKLE SUPPORT POWERED BY THE BOA CLOSURE SYSTEM
Descrition
ANKLE INJURIES
Ankle injuries are often considered sports injuries, but you don’t have to be an athlete to suffer an ankle injury. Sometimes, simply moving over an uneven surface is enough, which can cause the foot to misalign and become painfully sprained. Common injuries to the ankle joint include sprains, fractures, inflammation or rupture of the Achilles tendon. An ankle sprain can occur in one of three areas: medial, lateral and superior. The most common injury to the ankle joint is an excessive inversion, which is commonly referred to as an ‘ankle sprain’. The injury occurs when running on an uneven surface, incorrectly stepping or pivoting on the foot. It accounts for 85% of all ankle joint injuries. Specialised ankle braces, such as the AM-SX-02/CCA, are used to treat and prevent ankle injuries.
Product’s description:
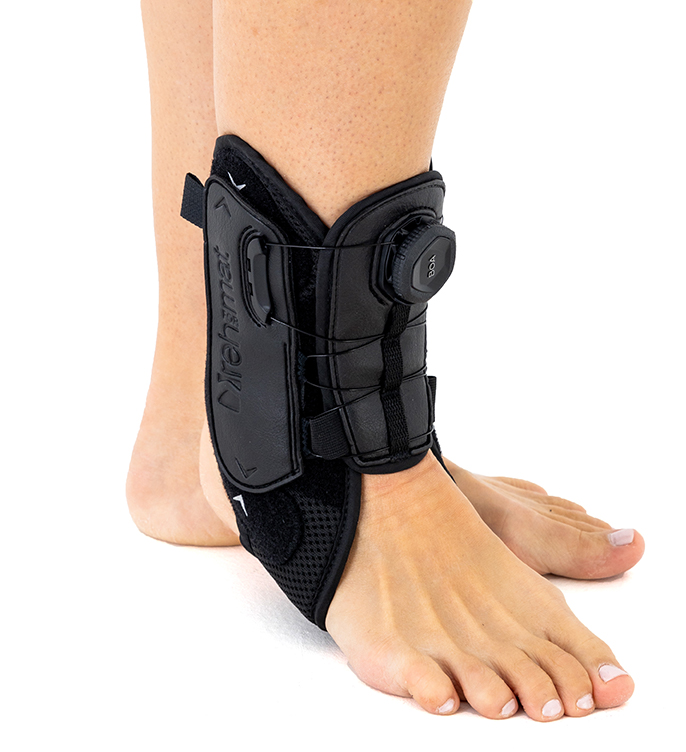
The AM-SX-02/CCA professional ankle brace with CCA BOA® compression System provides unique stabilisation of the ankle joint and protects against acute and chronic injuries. Our ankle brace with BOA® System provides optimum ankle support and reduces the risk of overusing. It provides comprehensive stabilisation during movement without causing discomfort on the skin.
The innovative open design ensures a perfect fit of the product to the ankle, both in the acute period when swelling occurs and prophylactically in physical activity.

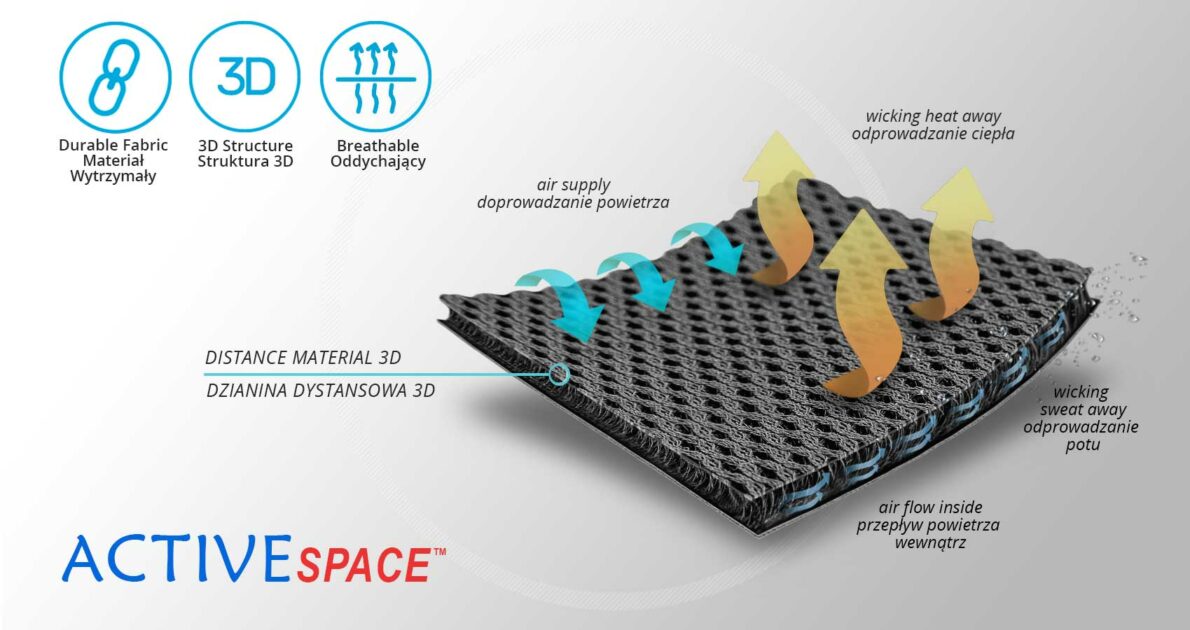
The AM-SX-02/CCA ankle brace is made from skin-friendly fully breathable ActiveSpace™ material, which guarantees all-day comfort and airflow, while offering significant ankle support during a variety of sport activities.
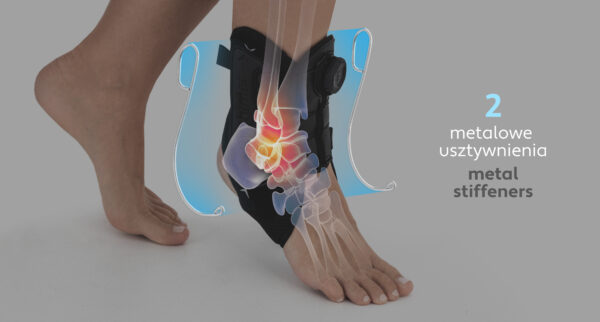
The ankle brace with BOA® system AM-SX-02/CCA features two metal lateral stabilizers that help stabilise the joint, while their anatomical shape prevents pressure on bony prominences and provides unparalleled support and comfort.
Featuring the innovative patented BOA® Compression System, the BOA® System provides a secure adjustment of the level of stability with an intuitive dial, even during intense physical activity.
The CCA compression system with BOA® is a quick fastening tool with a lightweight knob that is much more comfortable than a shoelace, allowing quick adjustment of elasticity and even distribution of pressure on the limb for comfort and convenience.
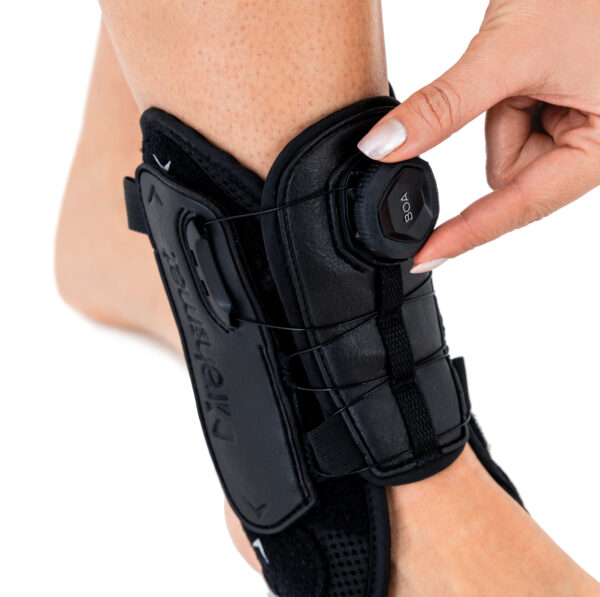
The compact design of the ankle brace allows it to be worn with any type of footwear, including sports and everyday comfort shoes.

Purpose of use:
- Chronic ankle instability
- Prophylactic talocrural support
- Prevention or treatment of acute ankle sprains
- Subtalar joint sprain
- Syndesmosis sprain
Sizes
| Size | Heel circumference | How to measure |
| M | 27-33 cm | 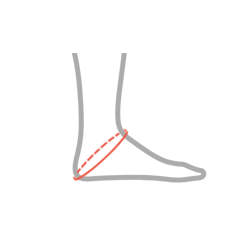 |
| L | 33-39 cm |
Fits for both ankles.
Total height of the orthosis:
M – L: 21 cm
Gallery
Technology
MATERIALS
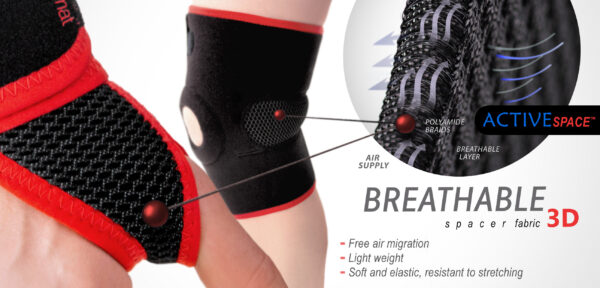
ActiveSpace™
ActiveSpace™ is a spacer, polyamide 3D lamination with high skin ventilation. It is very lightweight, consisted of 2 layers. Between them, we use polyamide braids. ActiveSpace™ is not elastic what improves stabilization. Inside the lamination, between 2 layers, the air flows freely, maintaining minimal water and moisture absorption. Waterproof material.
TECHNOLOGICAL SYSTEMS
BOA® Fit System
 DIAL IN TO FAST, EFFORTLESS, PRECISION FIT.
Delivering fit solutions purpose-built for performance, the BOA® Fit System is integrated in products across snow sports, cycling, hiking/trekking, golf, running, court sports, workwear, and medical bracing. The BOA® Fit System is engineered with high quality, durable materials that are rigorously field-tested for a micro-adjustable connection that’s built to perform. Each unique configuration is engineered for power without compromising precision in order to deliver a seamless connection between equipment and body. BOA®’s dial and laces are guaranteed for the lifetime of the product on which they are integrated.
DIAL IN TO FAST, EFFORTLESS, PRECISION FIT.
Delivering fit solutions purpose-built for performance, the BOA® Fit System is integrated in products across snow sports, cycling, hiking/trekking, golf, running, court sports, workwear, and medical bracing. The BOA® Fit System is engineered with high quality, durable materials that are rigorously field-tested for a micro-adjustable connection that’s built to perform. Each unique configuration is engineered for power without compromising precision in order to deliver a seamless connection between equipment and body. BOA®’s dial and laces are guaranteed for the lifetime of the product on which they are integrated.
TURN&GO

STIFFENINGS
Ankle joint wire stiffening
Setting up
Downloads
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.