Midnight

 Knee brace
Knee brace Anatomic joint 2RA
Anatomic joint 2RA Class I medical device
Class I medical device Durable
Durable Innovative
Innovative Latex-free
Latex-free OA orthosis
OA orthosis Recommended by specialists
Recommended by specialists Waterproof
WaterproofMIDNIGHT – Functional knee ACL support brace
Description
Anterior Cruciate Ligament (ACL) Injury
The anterior cruciate ligament of the ACL is one of the two cruciate ligaments, the key stabilising elements of the knee. It connects the femur and tibia together, providing anterior stabilisation of the tibia relative to the joint axis and preventing it from extending too far forward. Damage to the ACL ligament can occur in a number of different ways. The most common mechanism is a sudden rotational manoeuvre during sporting activity, as is commonly seen in football, basketball or skiing. The ligament can also be torn as a result of work injuries or car accidents.
At the time of injury, a characteristic ‘pop’ can sometimes be felt or heard. There is then pain and a reduction in range of movement, and the person is unable to continue physical activity. Immediate swelling of the knee occurs at the time of injury – within the first few hours – but its extent can be reduced by prompt knee cold therapy or immobilization.
There are different types of ACL ligament injury, but regardless of the grade of the injury, it is always necessary to support and stabilise the joint with a functional knee brace like MIDNIGHT.
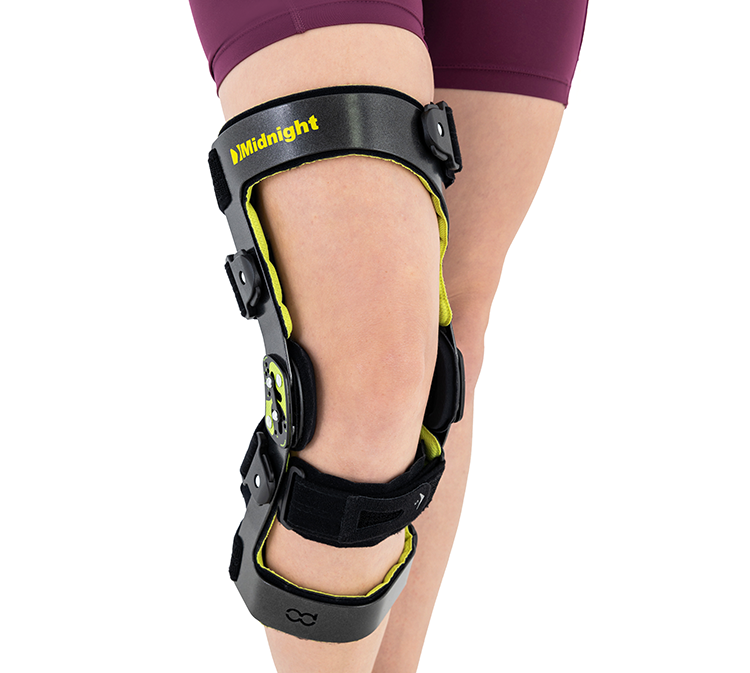
The MIDNIGHT knee brace is lightweight yet durable and provides professional support. The functional knee brace will provide adequate stabilisation both in the event of a joint injury and in chronic inflammation and arthrosis.
Our MIDNIGHT functional knee brace has been designed with the user’s comfort in mind. It is made from ultralight, anatomically shaped aluminium, providing maximum support and stabilisation with minimal product weight.
The thermoformed padding, the length of which can be adjusted individually, reduces pressure on the bony prominences and ensures comfort. It is equipped with skin-friendly silicone to prevent the product from slipping during use.
The brace has an anterior-anterior design, which makes it ideal for protecting the shin from forward translation, which occurs in cases of anterior cruciate ligament (ACL) injury. The aluminium frame design allows the brace to be used even in cases of advanced ligament damage. In addition, the MIDNIGHT knee brace keeps the joint in alignment, so it will prove useful for people with known knee valgus or knee varus, in order to prevent further progression of the deformity.
Once set, the circumferential range of the Velcro straps remains in place, even during prolonged use of the brace, and the quick pin fastening system ensures simple and intuitive application of the product.
This durable and lightweight knee brace is designed to provide the user with relief and safety. Functional knee brace MIDNIGHT is equipped with the 2RA Precision Pro hinges that provides an anatomical knee range of motion. The innovative ROLL-BACK technology provides an smooth anatomical knee motion. This solution allows the knee to be supported during flexion and extension, but also maintains constant contact between the articular surfaces of the thigh and shin, which allows the natural rotational movements of the joint to be maintained and supports the knee in any physiological range. Keeping the joint surfaces in constant physiological contact increases the proprioceptive effect of the knee, extremely important in the case of ligament damage.
The MIDNIGHT functional knee brace, with control of physiological joint mobility and ROLL-BACK technology, helps to provide optimal knee support for ACL cruciate ligament injuries. It also provides deep relief from osteoarthritis and patellar arthritis, knee pain, swelling, stiffness, injury, degeneration or other knee joint problems.
Purpose of use:
- Mild or severe ACL, MCL and LCL instability
- Rehabilitation after ACL reconstruction
- Mixed ligaments injuries
- Knee hyperextension

Sizes
| Size | (A) Thigh circumference 15 cm above the center of the patella | (B) Calf circumference 15 cm below the center of the patella | How to measure |
| S | 40 – 44 cm | 30 – 34 cm | 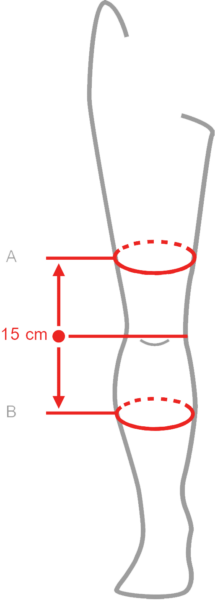 |
| M | 44 – 48 cm | 34 – 38 cm | |
| L | 48 – 52 cm | 38 – 42 cm | |
| XL | 52 – 56 cm | 42 – 46 cm |
Right and left leg specific.
Total length of the product:
S – XL: 39 cm
Gallery
Technology
MATERIALS
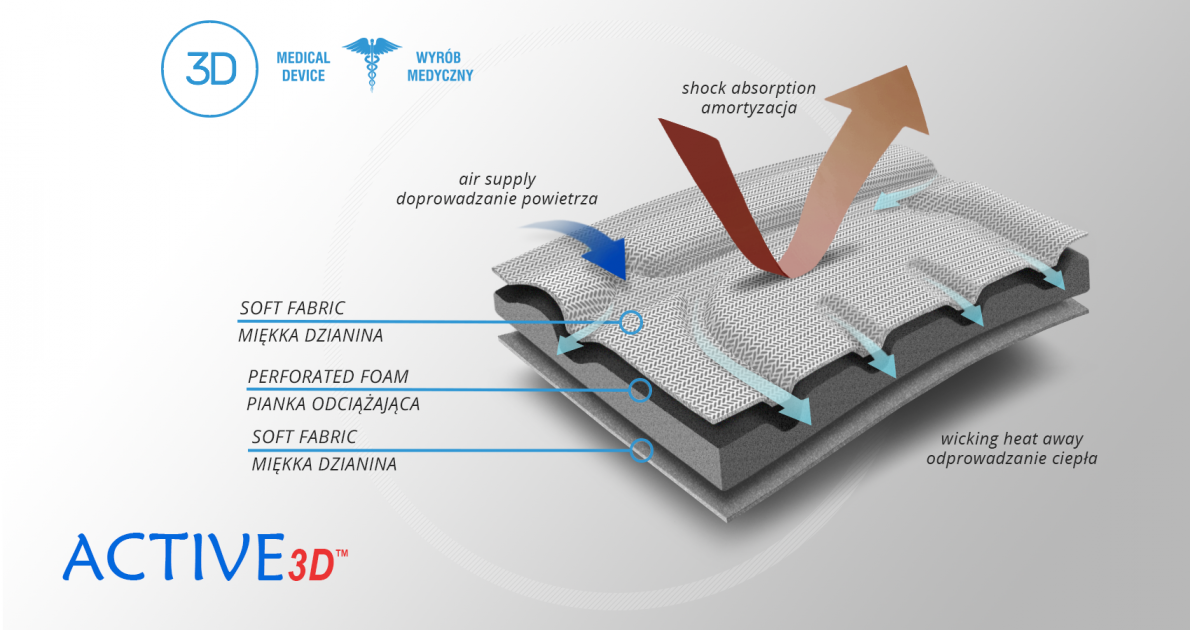
Active3D™
Active3D™ is thermoformed fabric made of special foamed, cell-closed designed material. It reduces the pressure on the body or any abrasions made by orthopaedic stays and aluminum splints. It is fully waterproof fabric and does not absorb sweat. It’s easy to clean. Due to its features, the fabric is an excellent product for making medical orthopaedic braces and orthoses. ACTIVE 3D™ has various external self-gripping layers. Our material has special, thermoformed properties and may be shaped according to the functional goals of the final braces.
STIFFENINGS
2RA PRECISION PRO HINGES
The 2RA Precision Pro splints advanced aluminium splints with an innovative hinge that provides an anatomical knee range of motion. The innovative hinge of the splints with ROLL-BACK technology provides an smooth anatomical knee motion. This solution allows the knee to be supported during flexion and extension, but also maintains constant contact between the articular surfaces of the thigh and shin, which allows the natural rotational movements of the joint to be maintained and supports the knee in any physiological range.
The hinges are manufactured with high quality of sanded aluminium, durable cover which what makes splints neutral to the influence of sweat and salt. Flexion and extension angle adjustment is possible with special Allen screws, which is included with all hinged braces. 2RA Precision Pro hinges provide wide ROM adjustment: knee flexion limit in 10, 30, 40, 60, 75 and 90 degrees and limit of knee extension in 10, 20, 30 and 40 degrees. There is also option to immobilize knee joint in full physiological extension.
This adjustment is carried out by means of special screws - the appropriate Allen key necessary for this operation is added to each product. The method of adjusting the flexion angle, prevents unauthorised adjustment of the clock.
PADDINGS
3D supports
3D relief supports are independent technical solutions to relieve the rigid elements of a given orthosis. These elements are made of supporting foams or EVA foam. These foams are connected with various types of skin-friendly materials and materials with an adhesive function. These pads have the appropriate shape and color adapted to the type of orthosis. They relieve both metal elements of orthoses, such as splints, stays, underwires and orthopedic drop locks, as well as other elements that should not come into direct contact with the patient's skin. These pads have an anatomical shape and are made of comfortable foam with proper hardness and elasticity, guaranteeing the proper therapeutic effect.
Setting up
Downloads
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.