AM-OSN-U-23

 Thumb brace
Thumb brace Wrist brace
Wrist brace Anatomic wrist splint
Anatomic wrist splint Class I medical device
Class I medical device Compression
Compression Innovative
Innovative Orthopedics
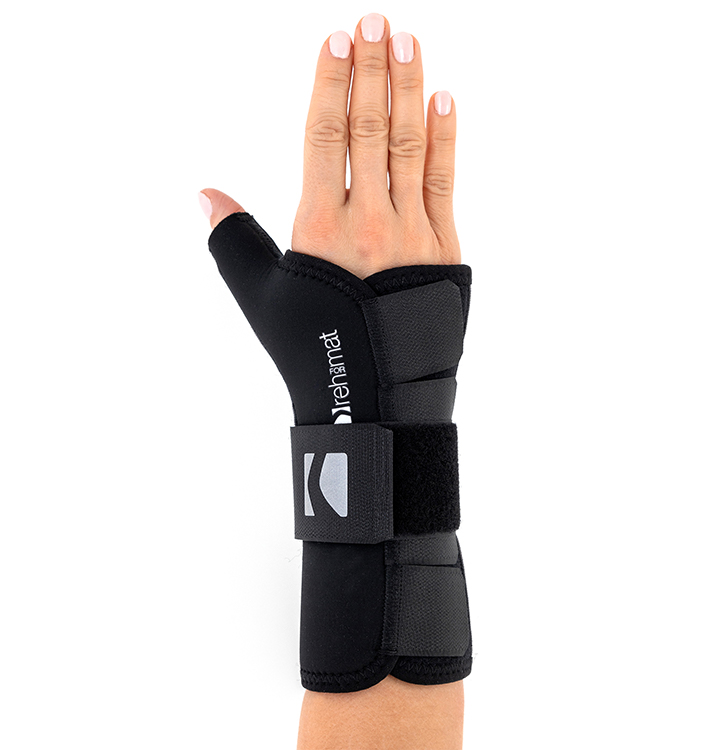
OrthopedicsAM-OSN-U-23 – WRIST BRACE WITH ABDUCTED THUMB
Description
THUMB INJURY
A thumb sprain is one of the most common injuries in which a ligament within the thumb joint is stretched or torn. In severe thumb sprains, the ligament may be partially or completely torn. Treatment mostly involves immobilization of the saddle joint in a physiological position and stabilization of the radiocarpal joint. For this purpose, the AM-OSN-U-23 professional wrist brace with thumb abduction will be useful.
Purpose of use:
The AM-OSN-U-23 wrist brace effectively immobilizes the radiocarpal joint and the thumb saddle joint in a neutral position. The product provides therapeutic compression, improving blood supply to the tissues and accelerating the healing process.
The brace has two immobilizers. The aluminum, anatomically shaped splint holds the wrist in a physiological position and reduces excessive tension on the soft tissues.

If a less stabilization is required, the aluminum splint can be removed and the brace can be used in a compression option.
The gentle lateral immobilization of the thumb stabilizes the saddle joint in a neutral position, prevents thumb adduction and improves ligaments regeneration.
Each brace can be freely adjusted and shaped according to the individual user’s needs.
The brace is made of lightweight, breathable UniPren™ for increased comfort.
Comfortable 3 Velcro closures for secure and professional stabilization.
Wygodne zapięcie w formie 4 rzepów, zapewnia bezpieczeństwo i profesjonalną stabilizację.
The AM-OSN-U-23 wrist and thumb brace works well for thumb sprains, saddle joint injuries, wrist dislocations, strains, scaphoid bone injuries, carpal tunnel syndrome, player’s thumb and De Quervain’s syndrome symptoms.
- Purpose of use:
- Sprained thumb
- Thumb dislocation
- Trigger thumb
- Keeper thumb
- Thumb arthritis
- Gamekeeper’s thumb
- de Quervain’s syndrome
- Stener lesion of the thumb
- Painful first carpometacarpal joint
Rozmiary
| Size | Wrist circumference (A) | Circumference at Thumb IP (B) | How to measure |
|
S |
13 – 15 cm | 5 – 6 cm | 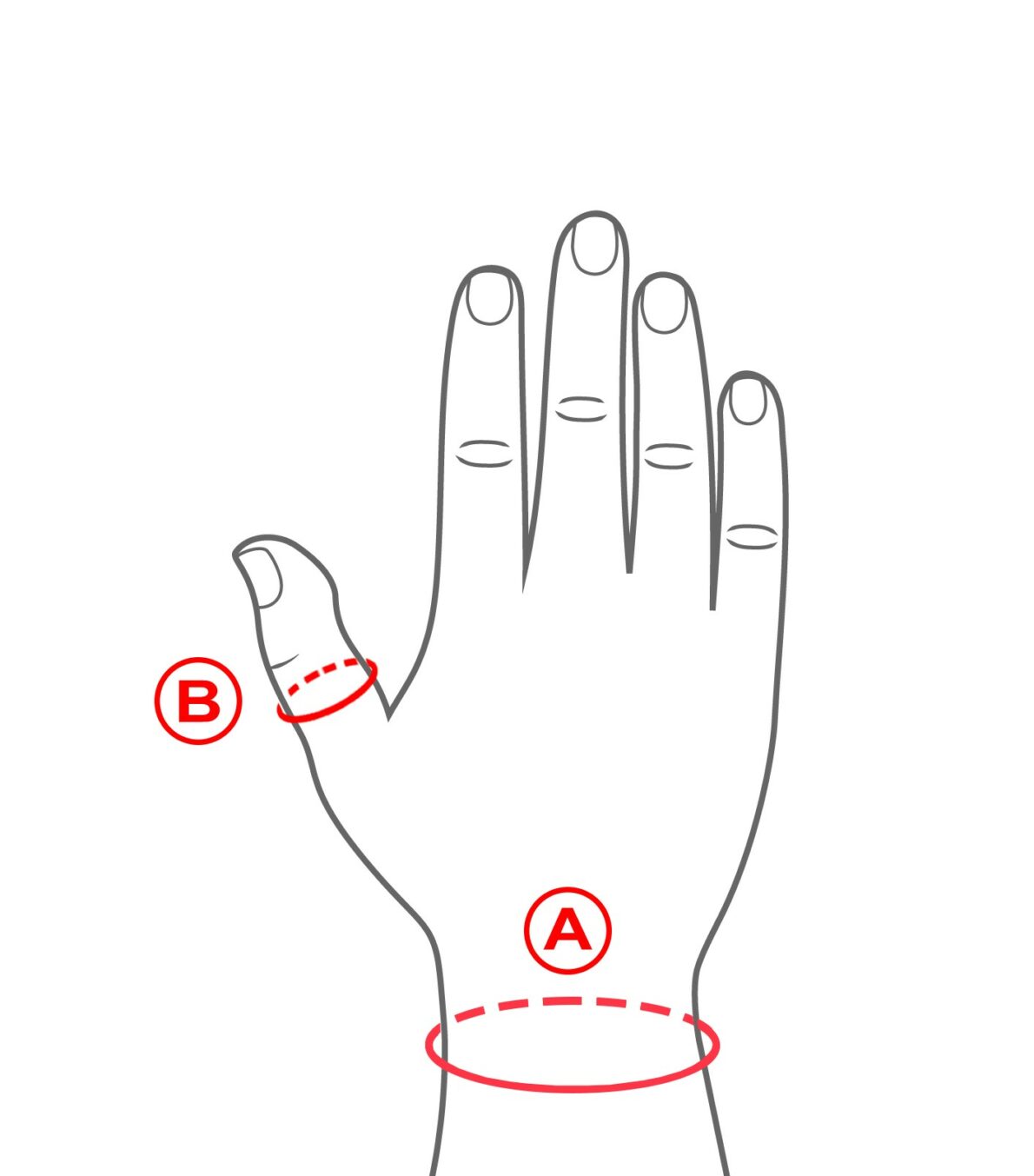 |
|
M |
15 – 17 cm | 6 – 7 cm | |
|
L |
17 – 20 cm | 7 – 8 cm | |
| XL | 20 – 23 cm | 8 – 9 cm |
Right and left wrist specific.
Total length of the product:
S-XL: 19 cm
Gallery
Technology
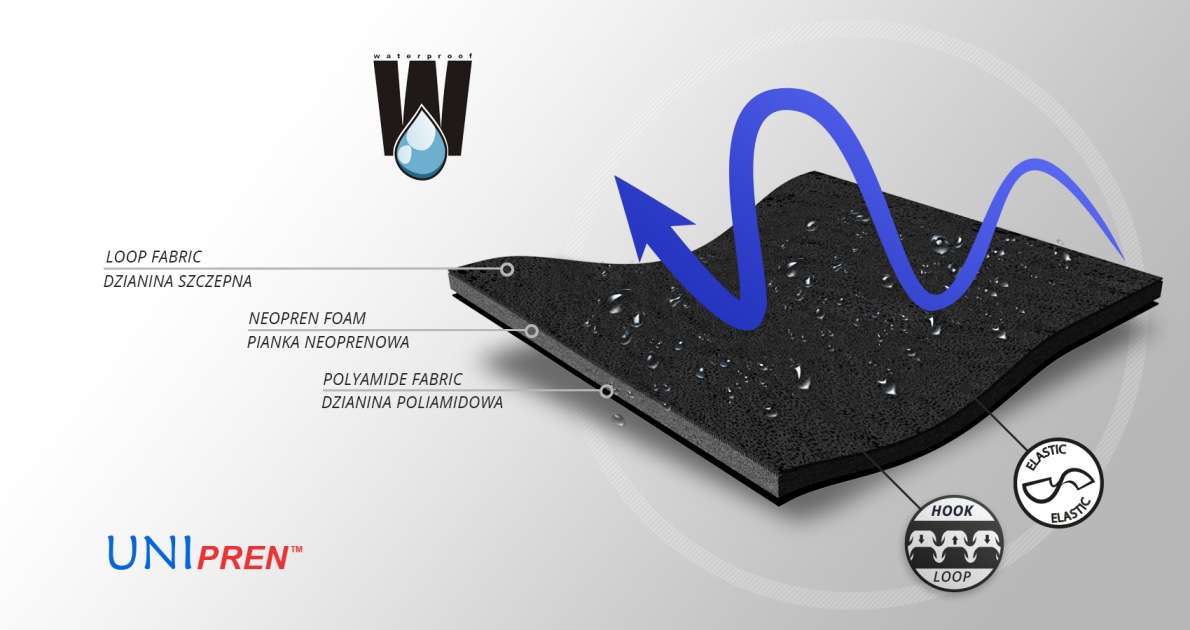
MATERIALS
UniPren™
UniPren™ is a universal 3-layer material consisting of an external elastic polyamide cover knit with a self-adhesive function, an internal neoprene foam core and an elastic jersey cover knit. This material is characterized by softness and very high flexibility. A very important advantage of this material is the fact that it is not a knitted product, it does not have thick fibers, so that the weaves of the material do not imprint on the patient's skin and do not cause abrasionsin places of high compression. Products made of UniPren™ are the strongest and most effective stabilizing orthoses available on the market. Self-adhesive function, the raw material makes it much easier to use.
STIFFENINGS
Palm strip
Profiled aluminum stays, spine stabilizers
These are splints and orthopedic stays of various thickness and width, which are made of various types of aluminum alloys. All these splints and stays, before mounting to a given orthosis, have been pre-profiled, which allows for fitting the product to the body of a specific patient. However, for the correct operation of the device, they should be precisely bent to the patient's body by an orthopedist, physiotherapist or orthopedist technician. Only this action guarantees the proper protection and support of the patient's body.

Setting up
Downloads
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.