Torso support EB-LK-01


HIGH DORSO-LUMBO-SACRAL BACK SUPPORT WITH NON-ELASTIC STRAPS
Description
The product is available while stocks last
Product description
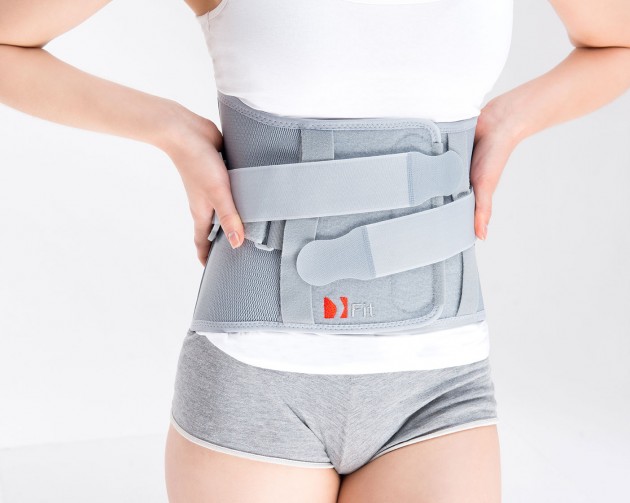
High dorso-lumbo-sacral back support Fit is the highest brace of our range, providing full support and stabilization of dorsal, lumbar and sacral part of the spine. The brace is anatomically shaped.
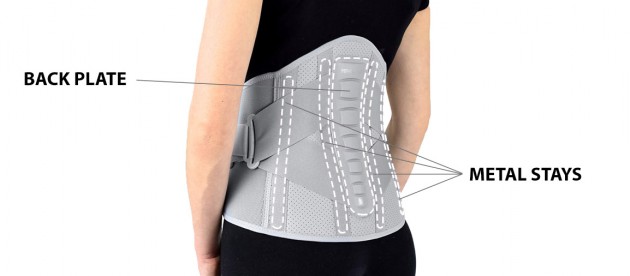
The Back part of support is made of innovative AirSanmed™.
The Back Support is provided with anatomically shaped metal stays (sweat-resistant coating), which quantity depends on the size; 4 or 6 metal stays. The orthosis incorporates semi-rigid Back Plate which function is to provide reinforcement of lumbar stabilization.
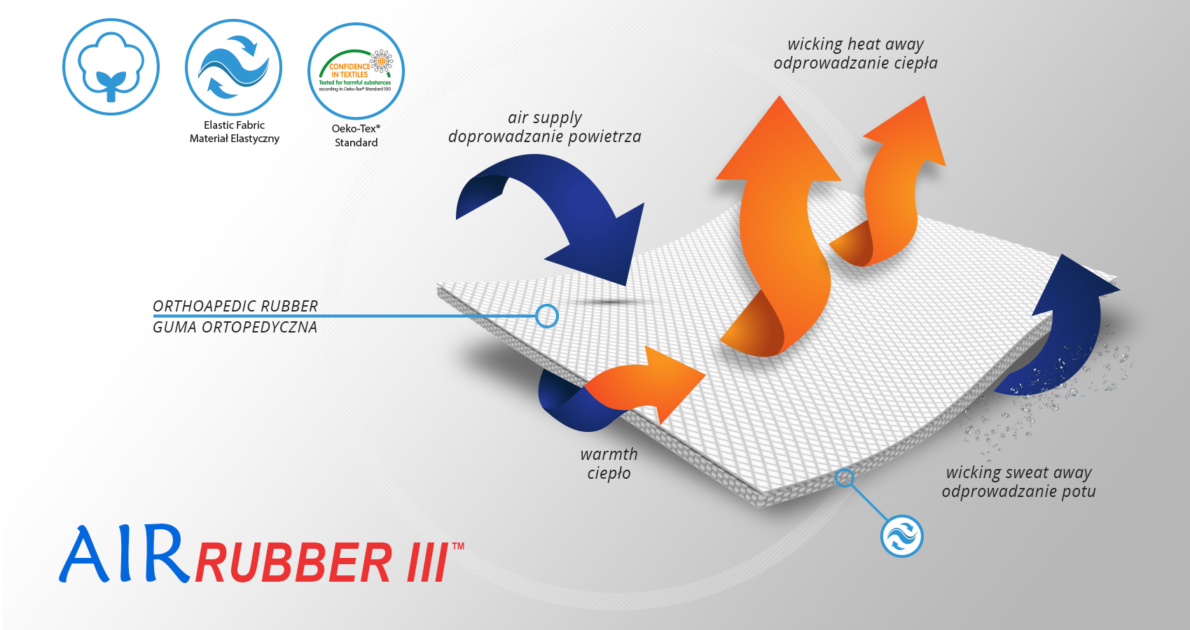
The lateral sides are made of strong multi-dimensional orthopaedic rubber AirRubber III™, which properties consists in 3D construction textile very breathable with unidirectional elasticity, offering compression and stabilization of area.
The back compression is obtained by two layers, non-elastic tensioners to regulate the compression and/or traction for the back medium dorso-lumbar region.
Easy to apply due to the closure system with hand grip pockets, specially developed for patient with hands disability.
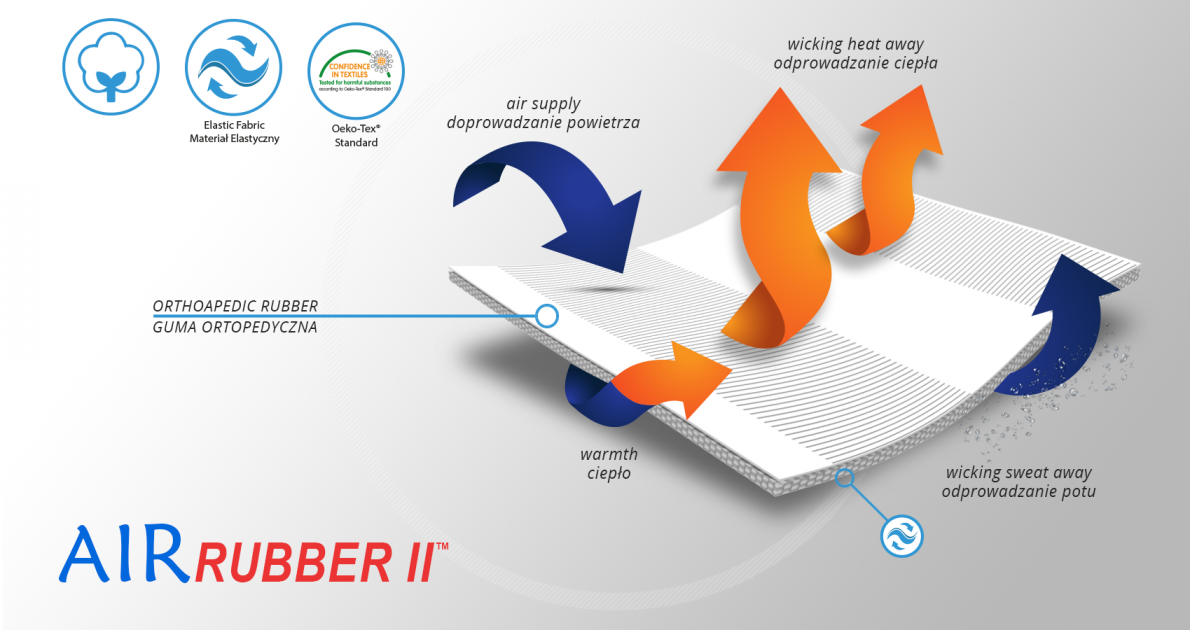
The regulation of the back compression is obtained by double fastening, non-elastic tapes made of AirRubber II™ and anatomically shaped system of buckles. The ends of closures are provided with plastic covers specially developed for patients with limited dexterity, such as osteoarthritis.
An asymmetric closure allows the perfect fitting on the patient body. The panel of closure is made of velcro laminate with comfortable semi-rigid foam located inside, additionally provided with two elastic whalebones. The system GRIP & OPEN makes the orthosis easy to assemble and disassemble.
Properties
– PATIENT-FRIENDLY PRODUCT – The lumber support is made of cotton orthopaedic rubber containing mainly cotton which is neutral to the skin.
– HIGH EFFECTIVENSS OF STABILIZATION – thanks to the anatomically shaped nibs and set of tightening tapes it was possible to achieve very effective stabilization.
– SAFETY – the front securing tapes increase the safety of usage of the product and eliminate the possibility of the uncontrolled opening of the device.
– COMFORT OF USAGE – the product is light and comfortable to use.
Indications
– Pre- and Post-Surgical Stabilization
– Degenerative Spinal pathologies
– Disc Hernia
– Spondylolithesis
– Spondylolysis
– Acute Back Pain
– Spine Instability
– Rehabilitation and Prevention
Sizes
Available sizes
| Size | Waist circumference | How to measure |
| S | 65-75 cm (25,6″-29,5″) |
 |
| M | 75-85 cm (29,5″-33,5″) |
|
| L | 85-97 cm (33,5″-38,2″) |
|
| XL | 97-110 cm (38,2″-43,3″) |
|
| XXL | 110-125 cm (43,3″-49,2″) |
|
| XXXL | 125-145 cm (49,2″-57,1″) |
Total height of the product: 33 cm (13″)
Technology
MATERIALS
AirRubber III™
AirRubber III™ has unidirectional elasticity. It can be stretched, increasing the length, not width, what improves compression. Between the braids air flows freely and skin can breathe. In addition in high compression, these rubber braids provide a gentle massage for skin. Our orthopedic rubber is very friendly for skin.
AirRubber II™
AirRubber II™ is breathable, perforated and has unidirectional elasticity. It can be stretched, increasing the length, not width, what improves compression. Between the braids air flows freely and skin can breathe. In addition in high compression, these rubber braids provide a gentle massage for skin. Our orthopedic rubber is very friendly for skin.
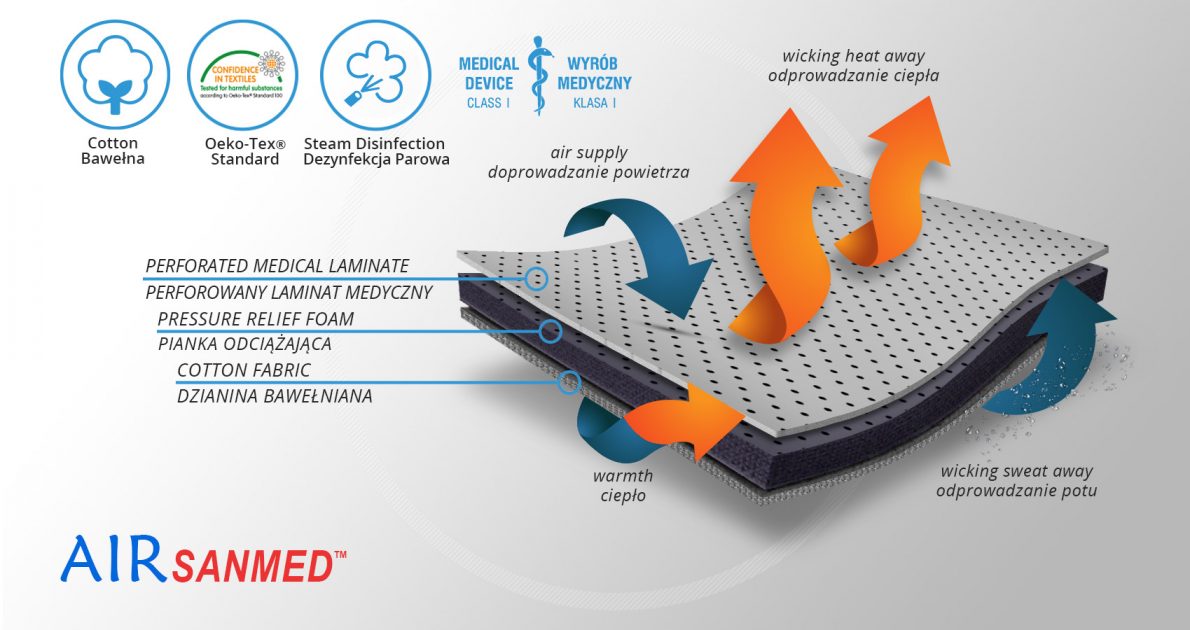
AirSanmed™
AirSanmed™ isn’t elastic what provides excellent stabilization. The skin has contact with cotton terry with Oeko-Tex Standard 100 certificate. There is semi-rigid perforated foam EVA inside that protect the skin against the metal splints influence. External side of the fabric is perforated medical laminate with antibacterial properties of Silver Zeolite. It provides long-term efficacy and prevents the most dangerous infectious microorganisms such as MRSA and E.coli. AirSanmed™ is in accordance with Health Minister`s ordinance of 3 November 2004 and Council Directive 93/42/EWG of 14 June 1993.
Setting up
Downloads
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.