Shoulders brace AM-SOB-05
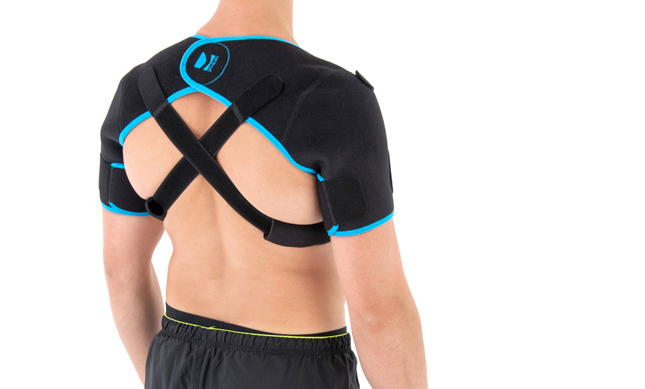

 Shoulder brace
Shoulder brace Class I medical device
Class I medical device Compression
Compression Double-sided
Double-sided Universal size
Universal size Waterproof
WaterproofUNIVERSAL SHOULDERS BRACE
Description
FROZEN SHOULDER
Frozen shoulder, also known as Duplay disease, is a closed set a lot of diseases connected with shoulder. The reason of the pain and limited motion can be stressed muscles, tendon injury or bursitis.
The main symptom of the frozen shoulder is pain. The pain can be little at the beginning and occurs only with rising limb above the arms. Over time, it can grow and is sensible with other motions. The pain can appear suddenly, e.g. after rapid movement and it’s often with swelling and limited range of motion.
The main reasons are:
1. stressed muscles
2. tendons injuries
3. bursitis
4. rhemautic diseases (RA, joint degeneration) or neurological (cervical and brachial plexus inflammation)
You can suffer from frozen shoulder after usual daily activities (e.g. window cleaning) or carrying heavy products. It can occurs after: repeated motions, uncomfortable sleeping position or intensive exercises.
The other reason can be also the rotator cuff injury (infraspinatus, supraspinatus, subscapularis and teres minor muscle). The tendons surround humeral head and joint capsule. The biceps injury can also cause frozen shoulder.
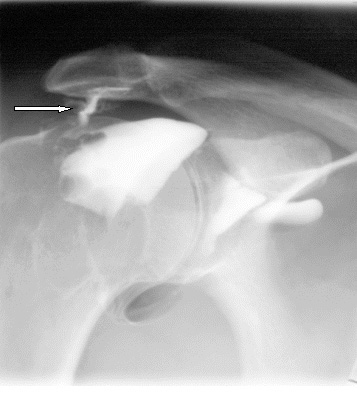
The frozen shoulder can become into Impingement Syndrome. This is result of inflammation caused by stressed tendon. The disease is often for young athletes, e.g. tennis player or swimmer.
Affected shoulder can’t be immobilized to stop the disease growing. Our innovative shoulders brace AM-SOB-05 supports the shoulder joints without immobilize them. The brace maintains the humerus in the acetabular. Our brace stops the disease progress and provides the pain relief.
Product’s description
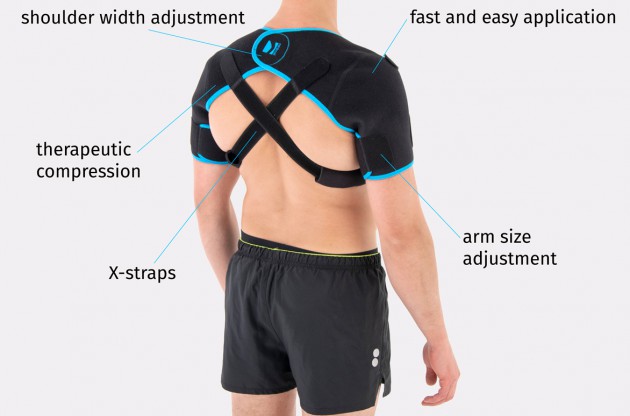
AM-SOB-05 shoulders brace is an universal product made of high quality fabric UniPren™. The brace provides therapeutic compression and reduces bruises. Our brace AM-SOB-05 is an excellent product for active people. It keeps the shoulders in correct position, prevents against the injuries. It supports the shoulders after conditions, reduces the inflammation and improves healing.
Shoulders brace AM-SOB-05 is made of two parts that are attached with the Velcro closure.
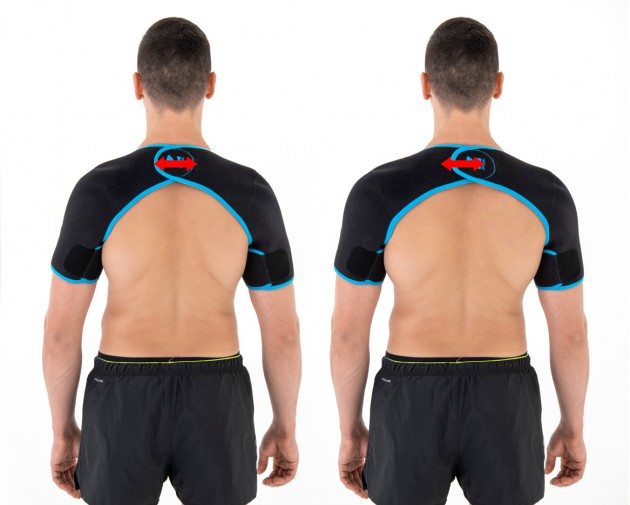
The arm sleeves are wrap around designed and you can fit the precisely with additional Velcro part.
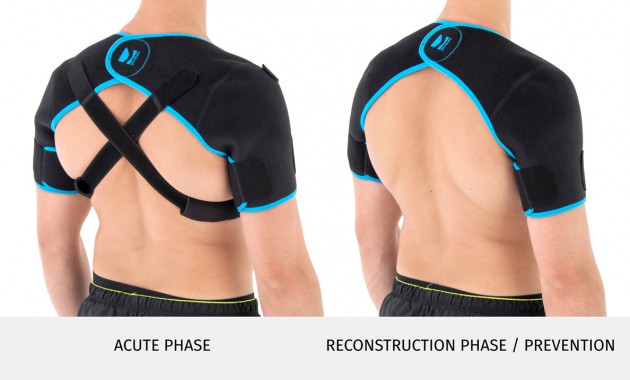
The brace has X-straps which maintain the shoulder in the correct positions, improve the posture and support upper limb. The X-straps should be used in acute swelling stage. When you start gently exercises in the remodeling stage, you can use AM-SOB-05 brace without the straps. Slightly exercises improves the collagen production and tissue recovery.
Of course, our AM-SOB-05 shoulder brace may be used also as a prevention against the injuries.
Purpose of use
– Frozen shoulder (Adhesive capsulitis)
– Shoulder impingement syndrome (subacromial impingement)
– Shoulder instability
– Lax capsule
– Aching shoulder
Sizes
| Size | The circumference of arms at the level of humeral head | How to measure |
| Universal | min 85 – max 95 cm min 33.5″ – max 37.4″ |
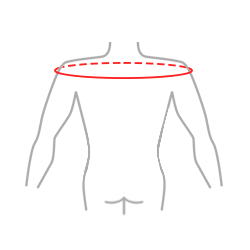 |
| X-Size | min 95 – max 110 cm min 37.4″ – 43.3″ |
Total length of the arm element:
Uni: 22 cm (8.7″)
X-size: 25 cm (9.8″)
Gallery
Technology
MATERIALS
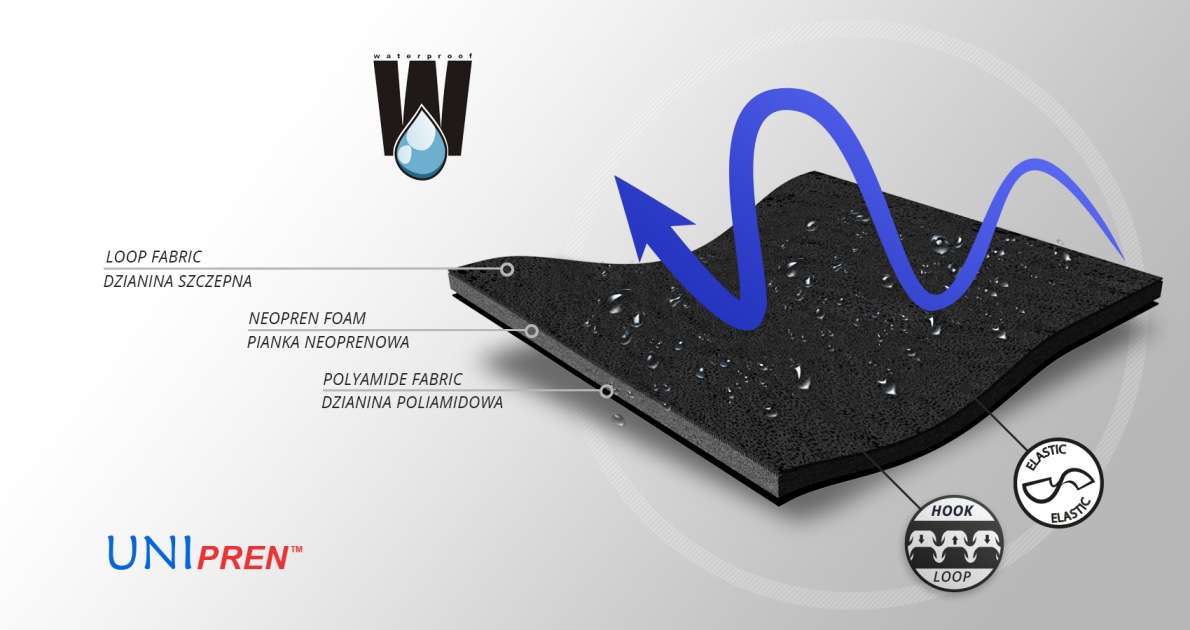
UniPren™
UniPren™ is a universal 3-layer material consisting of an external elastic polyamide cover knit with a self-adhesive function, an internal neoprene foam core and an elastic jersey cover knit. This material is characterized by softness and very high flexibility. A very important advantage of this material is the fact that it is not a knitted product, it does not have thick fibers, so that the weaves of the material do not imprint on the patient's skin and do not cause abrasionsin places of high compression. Products made of UniPren™ are the strongest and most effective stabilizing orthoses available on the market. Self-adhesive function, the raw material makes it much easier to use.
Setting up
Downloads
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.