Upper-extremity support AM-SOB-02
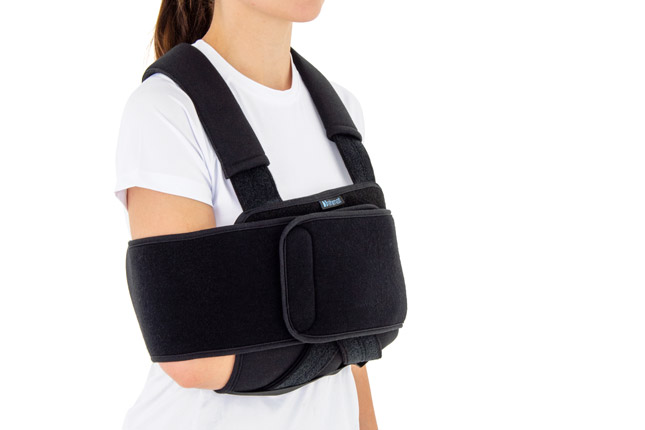

 Upper limb brace
Upper limb brace Breathable
Breathable Cast replacement
Cast replacement Class I medical device
Class I medical device Cotton
Cotton Double-sided
Double-sided ER
ER Machine washable
Machine washableTINOT
SHOULDER AND ELBOW IMMOBILIZER
Description
Our shoulder brace AM-SOB-02 consists of 2 elements: main part made of CottonComfort™ and additional immobilizer band for extra protection made of innovative fabric ActiveDistance II™.
Wide, free immobilizer band holds the arm securely to the body and provides extra stability.
Our shoulder brace AM-SOB-02 supports upper limb in case of dislocations, fractures and fatigues. The material is fully self gripping and easy to attach and you can fit the brace precisely. It provides stable immobilization and fast recovery.
Our universal shoulder brace AM-SOB-02 has innovative design. It can be used for both shoulders.
Moreover, the shoulder starps can be easily adjusted, so the brace can be fitted to each patient. The use of the tape in the lower part of the limb support element eliminates the risk of the straps moving and sliding during use.
Soft, adjustable shoulder strap covers improve the comfort of use and prevent pressure on the neck area.
The materials used in the product are extremely light and comfortable. Their airy and pleasant to the touch structure allows for complete oxygenation of the skin and significantly improves the convalescence period. The delicate foam does not compress the sensitive places and does not disturb the circulation. AM-SOB-02 brace can be successfully used not only during the day, but also as a night care.
Properties
- strong breathable material
- fully adjustable
- straps without strangling neck
- immobilizer band for extra protection
- maintenance of proper body hygiene – the patient can take the product off at any time and put it on again, it is possible to maintain the appropriate level of hygiene of the patient’s body without the need for other people.
Purpose of use
- shoulder dislocations
- after surgery immobilization
- soft tissues injuries in the area of shoulder
- after surgery as a protection
Sizes
| Size | Chest circumference | How to measure |
| Universal | min 70 – max 120 cm | 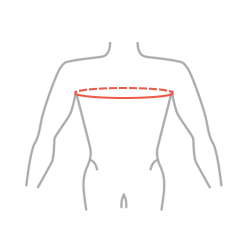 |
| X-SIZE | min 120 – max 150 cm |
Fits for both hands.
Gallery
Technology
MATERIALS
ActiveDistance II™
ActiveDistance II™ is an active 3-layered fabric made of non-elastic polyamide self-gripping layer, comfort foam and cotton terry. The last one layer is responsible for maintaining your skin dry. This material is skin-friendly and has the Oeko-Tex Standard 100 certificate. Self-gripping function of ActiveDistance II™ allows to fit the braces precisely.
CottonComfort™
CottonComfort™ is one of the most comfortable, antiallergic and ecological orthopedic fabric that we use in the production of our products. This raw material consists of a very comfortable polyurethane foam, laminated on one or both sides with a soft and comfortable cotton knit. The knit is made of natural cotton, without any chemicals containing formaldehyde. It is certified by Oeko-Tex® Standard 100, which guarantees safety to the patient's skin. The lamination process takes place without the use of any kinds of glue, but with the use of the flame method, which changes the surface of the polyurethane foam into a sticky surface to which the outer facings of the raw material stick. Thanks to the use of such a production technology, we do not unnecessarily introduce chemical chemicals into the structure of the raw material and we ensure a very high strength of such a connection. CottonComfort™ is used in orthoses in the most delicate places, so as not to cause skin irritation and so that the soft structure of the material tightly fills the free spaces between the orthosis and the patient's body.
STIFFENINGS
Stiffeners of thin ABS
Stiffeners made of a thin ABS plate are very light and protect the correct shape of the brace, but do not stiffen individual parts of the patient's body. The brace has the correct circumferential shape, but does not have the compensating and stiffening function of the protected joints and other protected parts of the body.

Setting up
Downloads
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.



























