Leg support U-PU
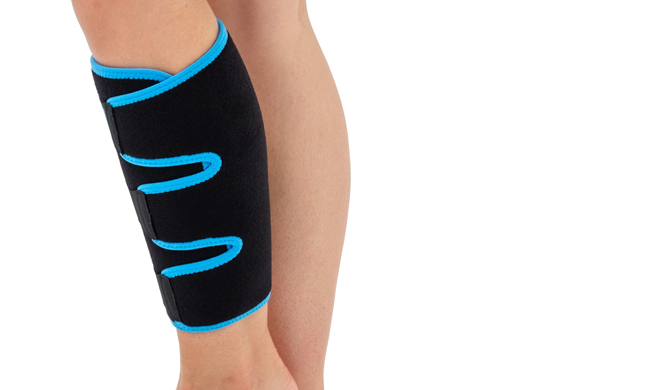

 Calf brace
Calf brace Class I medical device
Class I medical device Double-sided
Double-sided Latex-free
Latex-free Skin-friendly
Skin-friendly Universal size
Universal sizeUNIVERSAL SHIN BRACE
Description
The brace is made of UniPren™.
The brace is equipped with silicone anti-slp straps, which ensure proper positioning of the brace during use.
Properties
The support reduces pain of shin caused by overstress and old injuries. Maintenance of stable temperature and elastic pressure results in reduction of swelling, helps in healing of post-injury effusions and hematoma. Enables faster come back to training. Provides protection in contact sports. Detainment of warmth, preventing body overcooling and overheating, Comfort of use – the support contains no thick fibres which can cause abrasions if pressed firmly on patient’s skin. Simplicity of use- simple way to put the support on and take it off by the patient. The design of the support takes into account specific body shape and provides maximum comfort of use and no movement limitation for both women and men.
Purpose of use
– pain of shin caused by injury or overstress. Enables faster come back to training (e.g. after intensive cycling, indoor team sports, tennis, weight lifting);
– swelling of shin due to past injury and overstress;
– shin injury prevention – team indoor sports (e.g. handball, basketball)
Sizes
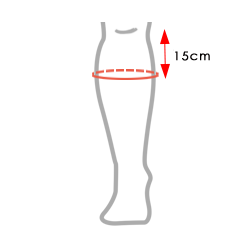
| Size | Calf circumference 15 cm from the knee | How to measure |
| Single size, fits both sides | min 25 cm – max 45 cm (min 9,8″-max 17,7″) |
 |
Total length of product: 27 cm (10,6″)
Gallery
Technology
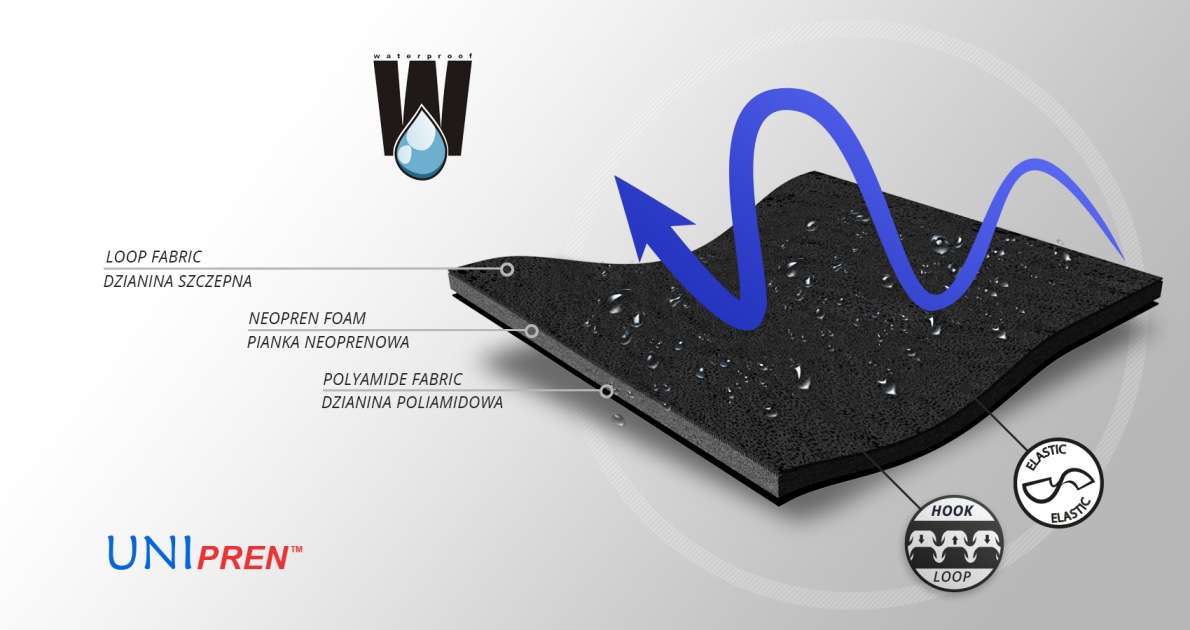
MATERIALS
UniPren™
UniPren™ is a universal 3-layer material consisting of an external elastic polyamide cover knit with a self-adhesive function, an internal neoprene foam core and an elastic jersey cover knit. This material is characterized by softness and very high flexibility. A very important advantage of this material is the fact that it is not a knitted product, it does not have thick fibers, so that the weaves of the material do not imprint on the patient's skin and do not cause abrasionsin places of high compression. Products made of UniPren™ are the strongest and most effective stabilizing orthoses available on the market. Self-adhesive function, the raw material makes it much easier to use.
Setting up
Downloads
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.