Lower limb support OKD-03
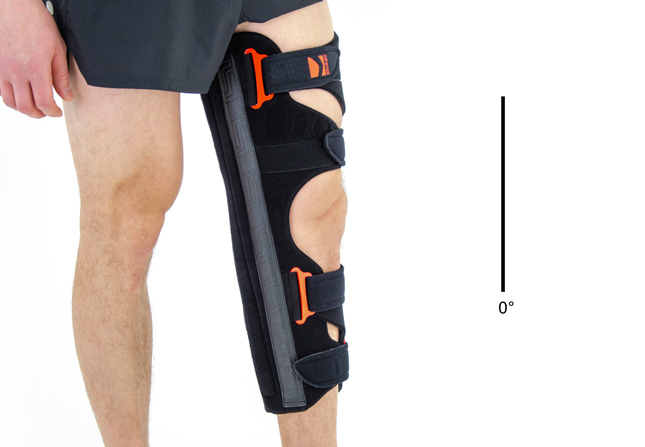

 Lower limb brace
Lower limb brace Cast replacement
Cast replacement Class I medical device
Class I medical device Double-sided
Double-sided Full range of adjustment
Full range of adjustment Orthopedics
Orthopedics Skin-friendly
Skin-friendly Universal size
Universal sizeLOWER LIMB EXTENSION IMMOBILIZER
Description
Lower limb extension immobilizer OKD-03 is made of Active3D™.
The brace is equipped with:
- Open and soft sleeve which enables to adjust the device to the diverse measurements of bottom limb.
- System of VELCRO stickers which assure proper adhesion the device to the body and more effective stabilization of limb.
- System of anatomically profiled aluminium splints with independent fixing system to brace’s sleeve.
- The kneecap protection with fastener adjustment
Such construction of article makes possible to adjust the device individually, easily and precisely to the patient’s body as well as assures sure stabilization of limb.
Purpose of use
Lower limb extension immobilizer should be applied in cases of:
- knee dislocation,
- knee twist and sprain,
- side instability of the knee joint,
- knee ligaments LCL, MCL and ACL injuries,
- knee ligaments reconstruction,
- other surgeries (orthopedic).
Sizes
| Size | (A) Thigh circumference 15 cm above the center of the patella | (B) Calf circumference 15 cm below the center of the patella | (C) Product length | How to measure |
| Single size | 40-65 cm (15,7″-25,6″) |
30-50 cm (11,8″-19,7″) |
55 cm (21,7″) |
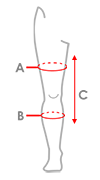 |
Total length of product: 55 cm (21.7″)
Gallery
Technology
MATERIALS
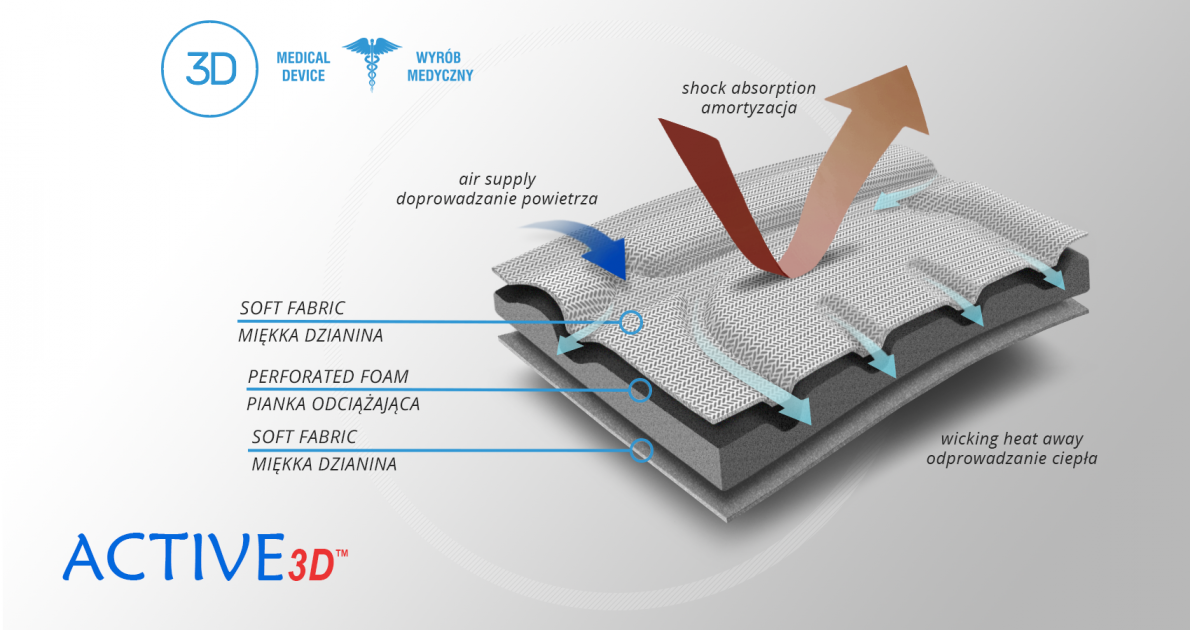
Active3D™
Active3D™ is thermoformed fabric made of special foamed, cell-closed designed material. It reduces the pressure on the body or any abrasions made by orthopaedic stays and aluminum splints. It is fully waterproof fabric and does not absorb sweat. It’s easy to clean. Due to its features, the fabric is an excellent product for making medical orthopaedic braces and orthoses. ACTIVE 3D™ has various external self-gripping layers. Our material has special, thermoformed properties and may be shaped according to the functional goals of the final braces.
STIFFENINGS
Flat aluminum stays
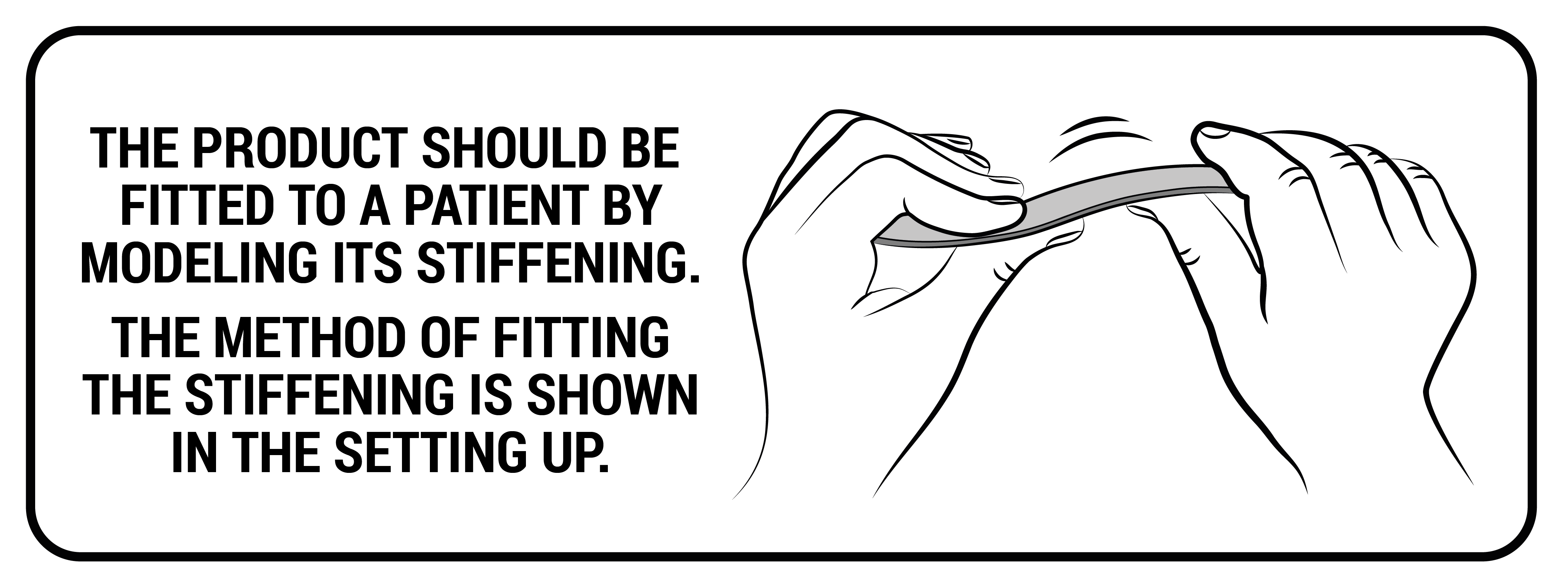
Aluminum stays are made of a special aluminum alloy that guarantees proper stiffness with minimal weight. They have rounded ends to prevent the damage of the product and come in various widths and thicknesses. The level of stabilization of the orthopedic device is defined by proper selection of the width and thickness of the aluminum stays. The stays can be pre-profiled or flat. They do not adapt to the shape of the patient's body, an individual adjustment of the orthosis is required by proper bending of the aluminum stays in the product. Thanks to this function, it is possible to correct the position of the patient's body or the secured joint.

Profiled aluminum stays
These are splints and orthopedic stays of various thickness and width, which are made of various types of aluminum alloys. All these splints and stays, before mounting to a given orthosis, have been pre-profiled, which allows for fitting the product to the body of a specific patient. However, for the correct operation of the device, they should be precisely bent to the patient's body by an orthopedist, physiotherapist or orthopedist technician. Only this action guarantees the proper protection and support of the patient's body.

PADDINGS
3D supports
3D relief supports are independent technical solutions to relieve the rigid elements of a given orthosis. These elements are made of supporting foams or EVA foam. These foams are connected with various types of skin-friendly materials and materials with an adhesive function. These pads have the appropriate shape and color adapted to the type of orthosis. They relieve both metal elements of orthoses, such as splints, stays, underwires and orthopedic drop locks, as well as other elements that should not come into direct contact with the patient's skin. These pads have an anatomical shape and are made of comfortable foam with proper hardness and elasticity, guaranteeing the proper therapeutic effect.
Setting up
Downloads
Accessories
ACCESSORIES / PRODUCTS TO BE USED WITH
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.