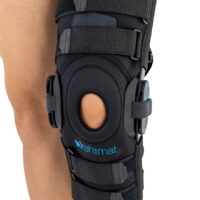
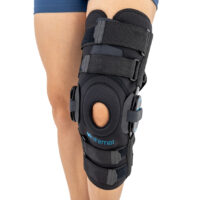
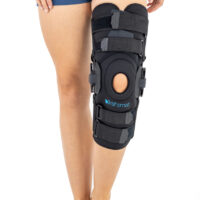
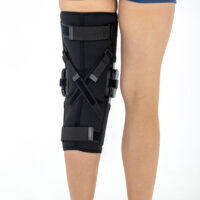
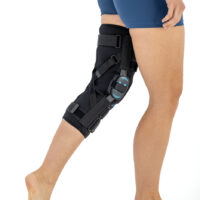
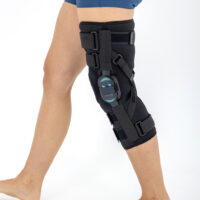
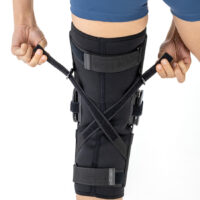
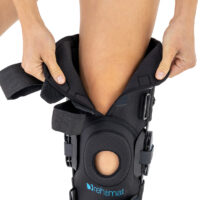
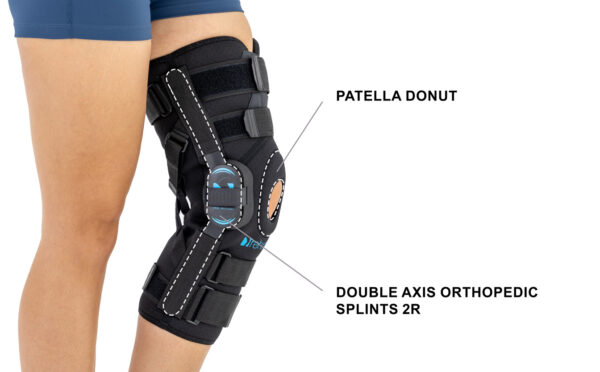
AM-OSK-ZL/2R-02 is the closed brace, made of innovative ActivePren™.
It has four independent sets of Velcro straps. The device is provided with 2-axial sides splints, which are made of high quality alloy of aluminium coated with permanent powdery cover. Length of splints and their system which is arranged in a shape of X letter, it allows to adhere perfectly to the limb and also enable to stabilize the joint excellently. The splints are equipped with special drop lock, which allows for flexion and extension adjustment in every 20°. Additionally, the brace holds special crucial rear fastener, which is the best protection of PCL (posterior cruciate ligament). Such precise adjustment of the device facilitates the process of rehabilitation of injured knee joint.
To achieve better fitting and limb stabilization we have provided anatomic splint 2R. It allows to maintain the hinges in the axial knee joint. This kind of splints is universal so it is possible to use it for both legs.
Purpose of use
AM-OSK-ZL/2R-02 brace should be applied in cases of:
- knee dislocation,
- knee twist and sprain,
- side instability of the knee joint,
- knee ligaments LCL, MCL, ACL and PCL injuries,
- after surgeries (orthopedic).
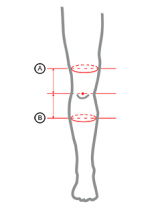
| Size |
(A) Thigh circumference 15 cm above the center of the patella |
(B) Calf circumference 15 cm below the center of the patella |
How to measure |
| M |
44-48 cm
(17,5″-18,9″) |
34-38 cm
(13,6″-15″) |
 |
| L |
48-52 cm
(19,1″-20,5″) |
38-42 cm
(15,2″-16,5″) |
| XL |
52-56 cm
(20,7″-22″) |
42-46 cm
(16,7″-18,1″) |
| 2XL |
56-60 cm
(22,2″-23,6″) |
46-50 cm
(18,3″-19,7″) |
Total length of product: 44 cm (17,3″)
MATERIALS
ActivePren™
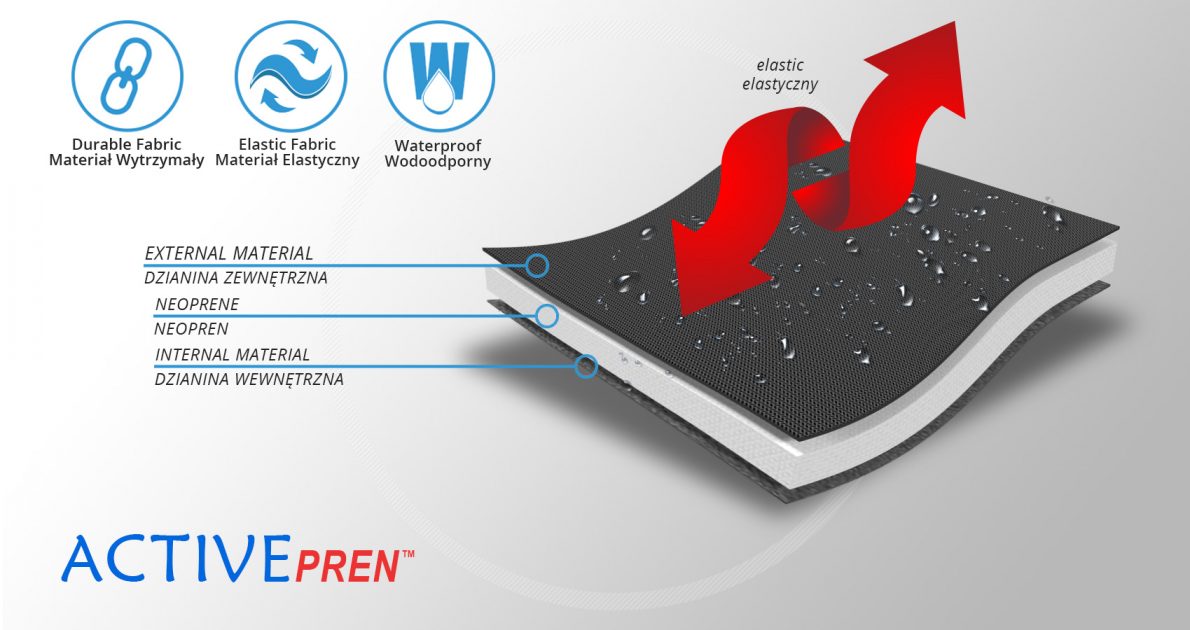
ActivePren™ is an active three-layer material consisting of two elastic jersey cover fabrics and a core made of neoprene foam. This material is characterized by softness and high flexibility. A very important advantage of this material is the fact that it is not a knitted product, it does not have thick fibers, so that the weaves of the material do not imprint on the patient's skin and do not cause abrasionsin places of high compression. Products made of ActivePren are the strongest and most effective stabilizing orthoses available on the market.
STIFFENINGS
Splint 2R
Splints 2R – double axis polycentric splints, with rack gear which reflects the anatomic knee movement. Such splints are used in all knee joint braces where apart from angle adjustment lateral joint stabilization is also important. The splints are manufactured with high quality of aluminium alloy with sanded, durable cover which is neutral to the influence of sweat and salt. Required flexion and extension angle in every 20° is set by using the special pins. The splints are equipped with special oval covers to prevent the pins from falling out as well as from changing of the angle by unauthorized people. Standard length of the splints is 390 mm, but the braces with longer splints are additionally equipped with lateral stabilization of the knee joint. The splint and its design are patented in European Union by Reh4Mat Company.



PADDINGS
Patella silicone support
A whole series of silicone supports made in various shapes and colors to relieve the patella or to stabilize it. These pads have appropriate shapes and protrusions, thanks to which the orthosis perfectly fulfills its function. These pads have an anatomical shape and are made of silicone with the appropriate hardness and elasticity, guaranteeing the proper therapeutic effect.
Patella stabilizers
Relief stabilizers of various shapes made in 2D technology. They are made of relieving foams connected on one side with a fabric friendly to the patient's skin, and on the other with a gripper, thanks to which the stabilizer can be attached to the adhesive element of the orthosis. Thanks to such a structure, these pads do not have to be sewn into the orthosis, and they can simply be fastened to it from the inside. These elements have an anatomical shape and are made of comfortable foam with proper hardness and elasticity, which guarantees the proper therapeutic effect.
Rubber supports


 Knee brace
Knee brace Anatomic patella donut
Anatomic patella donut Cast replacement
Cast replacement Class I medical device
Class I medical device Latex-free
Latex-free Polycentric joint 2R
Polycentric joint 2R
 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.