Lower-extremity support AS-SKL/F


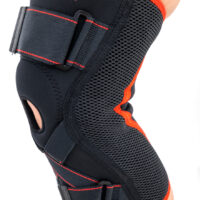
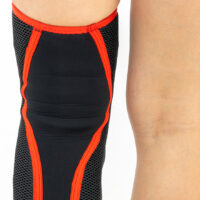
 Knee brace
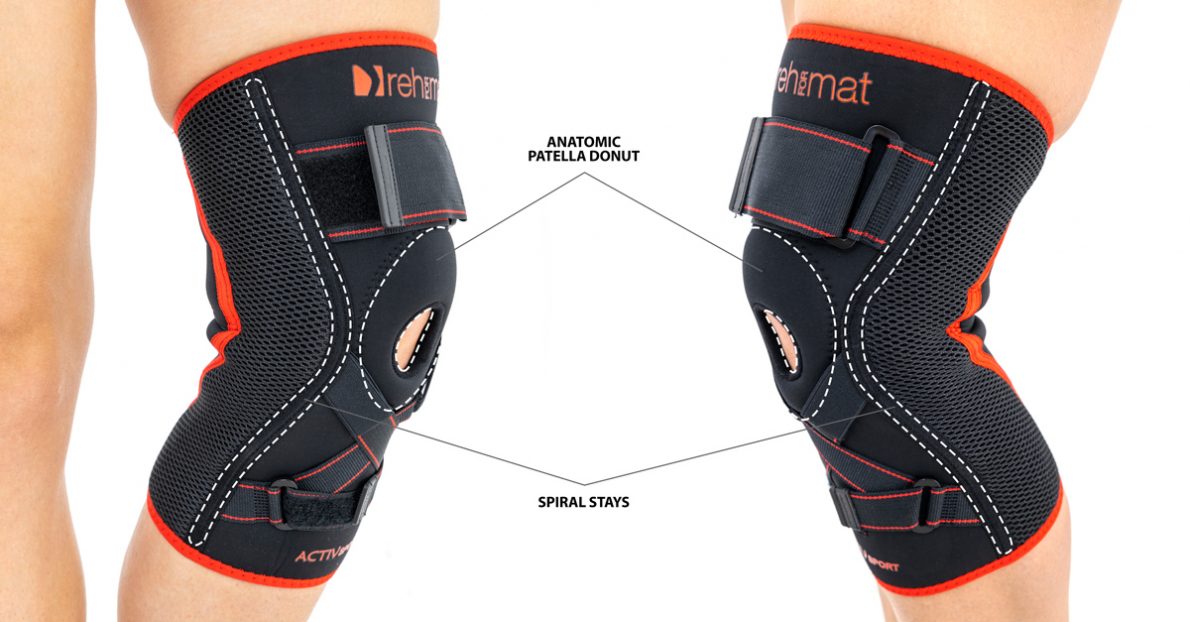
Knee brace Anatomic patella donut
Anatomic patella donut Class I medical device
Class I medical device Double-sided
Double-sided Durable
Durable Skin-friendly
Skin-friendly Spiral bonings
Spiral boningsACL ANATOMIC KNEE BRACE WITH ORTHOPAEDIC STAYS
Description
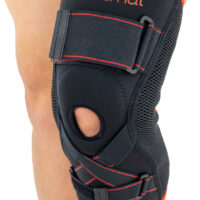
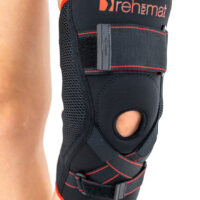
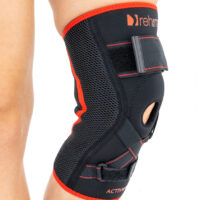
Knee brace AS-SKL/F belongs to the 4SPORT group. It consists of lateral stays, which protect the knee joint, VELCRO tapes and ACL support. The brace is made of innovative, compression and skin-friendly fabrics called ActivePren™ and ActiveSpace™.
The brace, thanks to its orthopaedic stays, stabilizes the knee joint. Furthermore, it supports the muscles insertions and has analgesic properties by stable compression. The design of the fabric enables use of the support even in extreme conditions e.g. sport activities.
Purpose of use
AS-SKL/F brace should be applied in cases of:
- stressed knee joint,
- knee twist and sprain,
- knee dislocation,
- little side instability of the knee joint.
Sizes
| Size | Knee circumference | How to measure |
| S | 30-34 cm (11,8″-13,4″) |
 |
| M | 34-38 cm (13,6″-15″) |
|
| L | 38-42 cm (15,2″-16,5″) |
|
| XL | 42-46 cm (16,7″-18,1″) |
|
| XXL | 46-50 cm (18,3″-19,7″) |
S – last items
Total length of the product: 31 cm (12,2″)
Gallery
Technology
MATERIALS
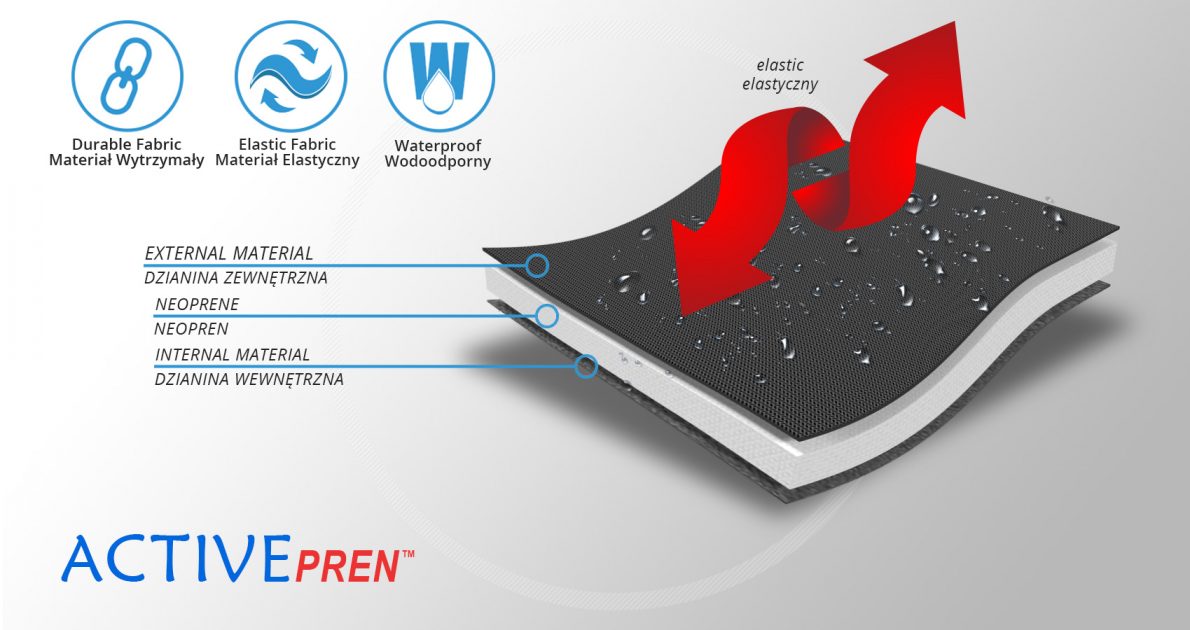
ActivePren™
ActivePren™ is an active three-layer material consisting of two elastic jersey cover fabrics and a core made of neoprene foam. This material is characterized by softness and high flexibility. A very important advantage of this material is the fact that it is not a knitted product, it does not have thick fibers, so that the weaves of the material do not imprint on the patient's skin and do not cause abrasionsin places of high compression. Products made of ActivePren are the strongest and most effective stabilizing orthoses available on the market.
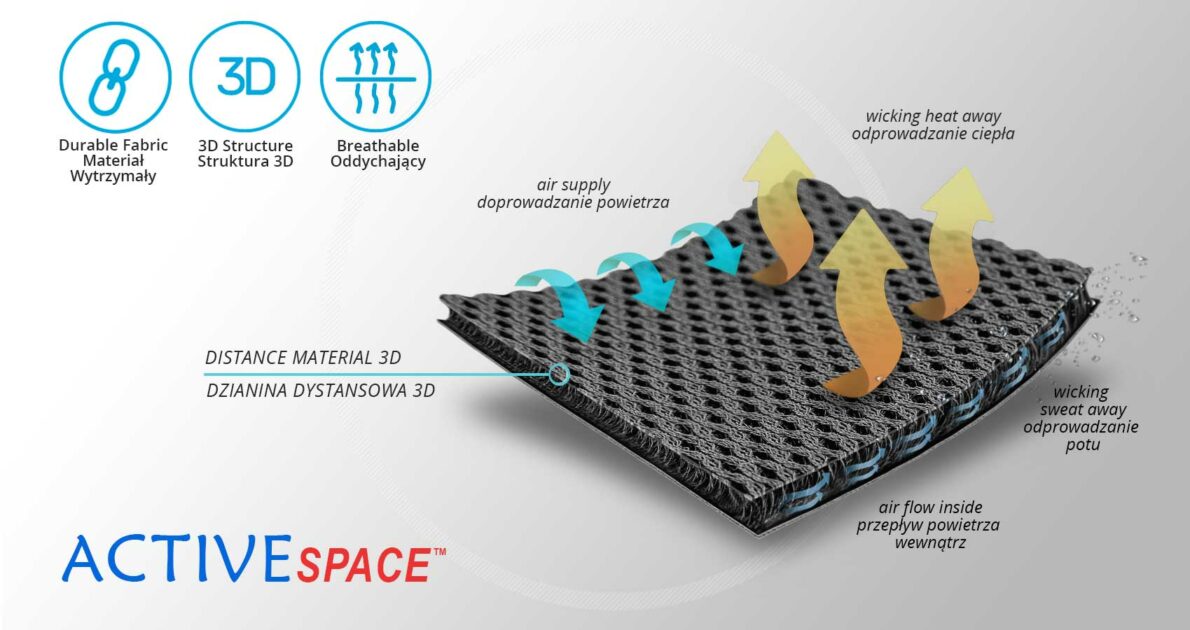
ActiveSpace™
ActiveSpace™ is a spacer, polyamide 3D lamination with high skin ventilation. It is very lightweight, consisted of 2 layers. Between them, we use polyamide braids. ActiveSpace™ is not elastic what improves stabilization. Inside the lamination, between 2 layers, the air flows freely, maintaining minimal water and moisture absorption. Waterproof material.
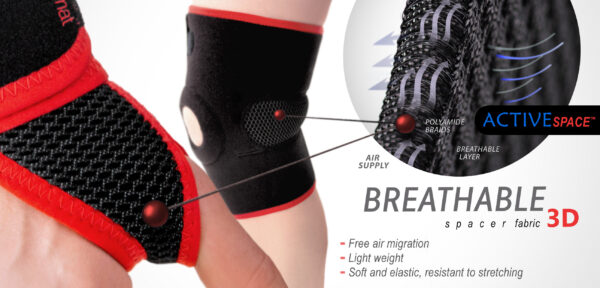
STIFFENINGS
Spiral boning
Our orthopedic spiral boning is a thin wire, coiled into a spring and flattened. We use boning of various widths and wire thicknesses, which determines the strength of their stabilization. Each spiral boning has two specially profiled fittings to prevent damage of the orthosis. They are galvanically protected against corrosion by the galvanizing process, so they are resistant to water, moisture and sweat. Products equipped with bonings can be washed without removing them from the orthosis. They work in every direction, perfectly adjusting to the user's body and have a shape memory function, thanks to which they always return to their original profile. This function causes the spiral boning in the orthosis to stabilize the swollen limb immediately after the injury and after the swelling has come off.

PADDINGS
Patella stabilizers
Relief stabilizers of various shapes made in 2D technology. They are made of relieving foams connected on one side with a fabric friendly to the patient's skin, and on the other with a gripper, thanks to which the stabilizer can be attached to the adhesive element of the orthosis. Thanks to such a structure, these pads do not have to be sewn into the orthosis, and they can simply be fastened to it from the inside. These elements have an anatomical shape and are made of comfortable foam with proper hardness and elasticity, which guarantees the proper therapeutic effect.
Setting up
Downloads
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.