Neck support AM-KM

 24h therapy
24h therapy Class I medical device
Class I medical device Efficiency
Efficiency ER
ER Skin-friendly
Skin-friendlySOFT ORTHOPAEDIC COLLAR
Description
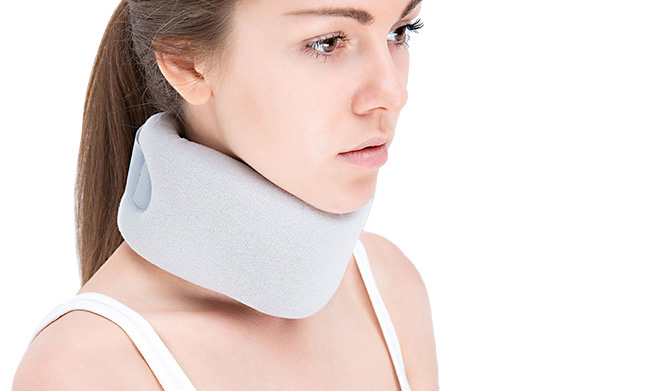
Orthopedic collar, Schanza type, made of polyurethane foam, covered with orthopedic cotton fabric. Fixed with VELCTO tape. The collar stabilizes and releases pressure form the spine in the neck area, releases stress from neck muscles.
Purpose of use
– After injury of the neck section of the spine (sprains and bruises)
– In rehabilitation after spinal fracture in prophylactic or surgery treatment
– In severe and chronic pain of the neck and shoulders due to the injury, overstress or degenerative changes
– In degenerative changes of the neck section of the spine combined with neurological symptoms (neck radiculopathy, paresis of upper limbs, balance disturbance, vertigo)
– In headaches and tympanophonia due to neck problems
– In torticollis due to different reasons
Contraindications
– Too tight application of the support may lead to increase of the problems, too loose application- luck of expected function
– Difficulty in breathing and discomfort, increase of existing problems- may limit or make impossible the use of the support (especially in case of overweight patients, with thyroid problems and increased neurotic symptoms) – after consulting with the leading doctor.
– It is necessary to control the correctness of the use of the support in case of children and patients with memory disorder or mental problems
– In case of skin changes in the place where the support touches the skin (abrasions, injuries, eczema) – use of the support is possible after a dressing is applies
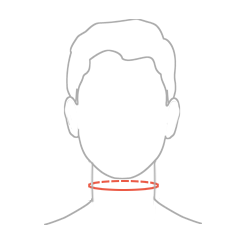
Sizes
| Size | Neck circumference | Available in heights | How to measure |
| S | 31-36 cm (12,2″-14,2″) |
8,10,12 cm (3.1″, 3.9″, 4.7″) |
 |
| M | 36-42 cm (14,2″-16,5″) |
||
| L | 42-48 cm (16,5″-18,9″ |
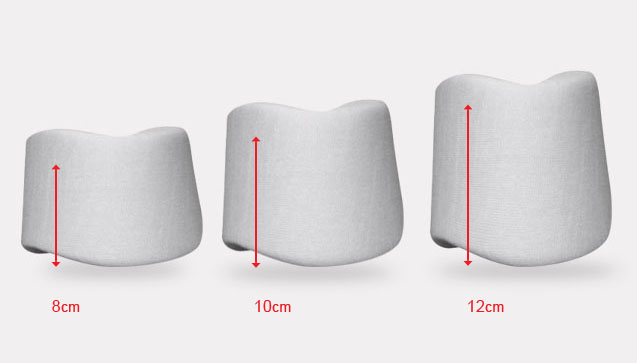
All sizes available in 3 heights: 8, 10, 12cm
Setting up


Gallery
Setting up
Downloads
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.

















