SPEEDERO/CCA


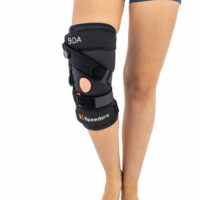
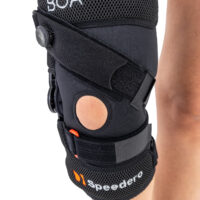
 Knee brace
Knee brace Class I medical device
Class I medical device Durable
Durable Efficiency
Efficiency Innovative
Innovative OA orthosis
OA orthosis Orthopedics
Orthopedics Skin-friendly
Skin-friendlySPEEDERO/CCA
Description
Sizes
| Size | (A) Thigh circumference 15 cm above the center of the patella | (B) Calf circumference 15 cm below the center of the patella | How to measure |
| S | 40 – 44 cm | 30 – 34 cm | 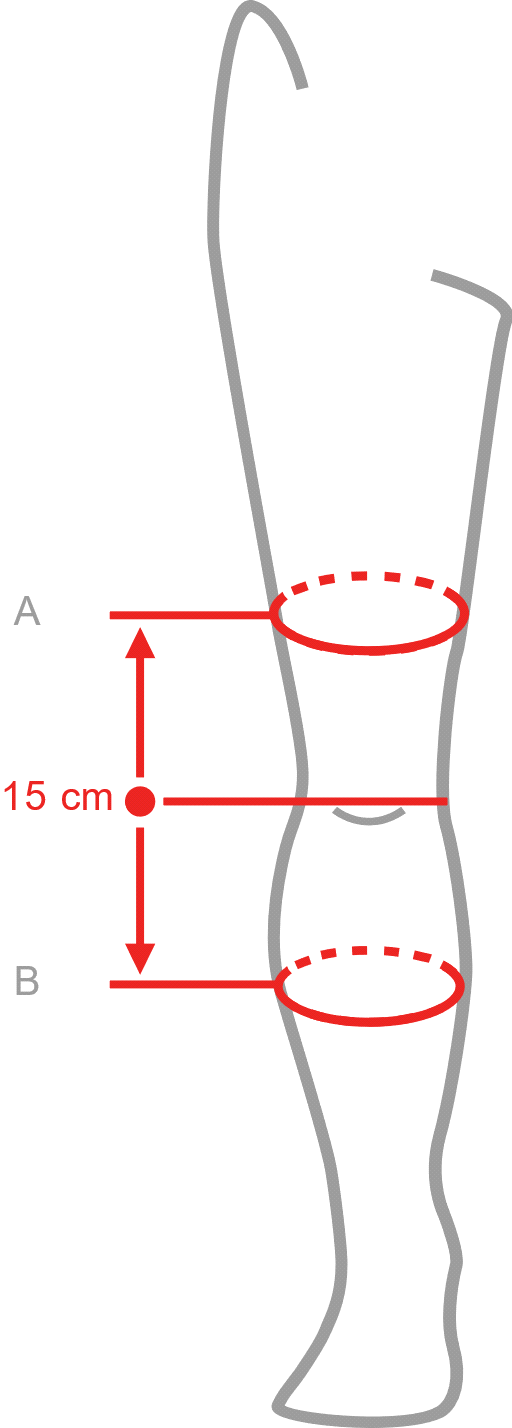 |
| M | 44 – 48 cm | 34 – 38 cm | |
| L | 48 – 52 cm | 38 – 42 cm | |
| XL | 52 – 56 cm | 42 – 46 cm | |
| 2XL | 56 – 60 cm | 46 – 50 cm |
Right and left leg specific.
Total length of the product: 35 cm
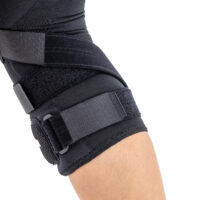
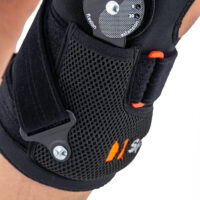
Gallery
Technology
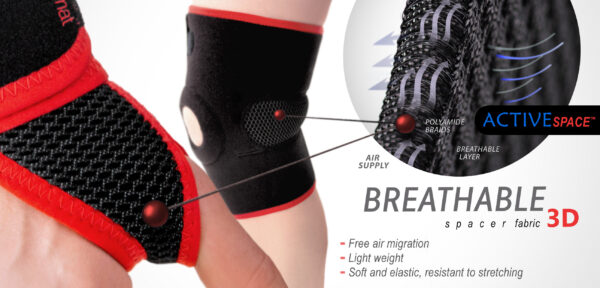
MATERIALS
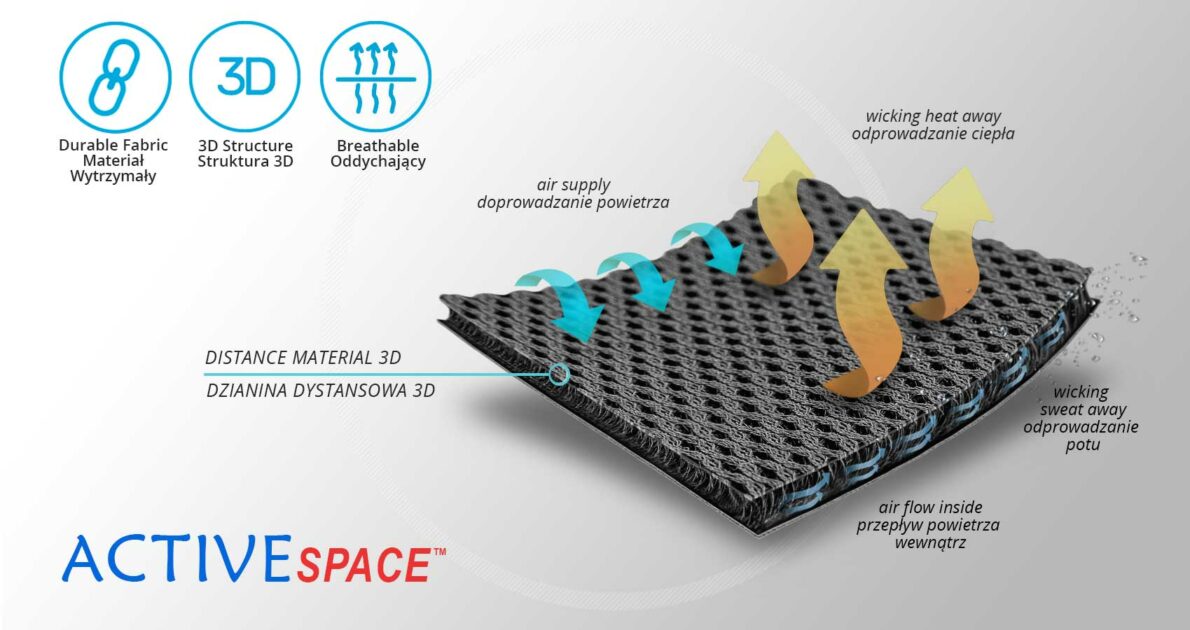
ActiveSpace™
ActiveSpace™ is a spacer, polyamide 3D lamination with high skin ventilation. It is very lightweight, consisted of 2 layers. Between them, we use polyamide braids. ActiveSpace™ is not elastic what improves stabilization. Inside the lamination, between 2 layers, the air flows freely, maintaining minimal water and moisture absorption. Waterproof material.
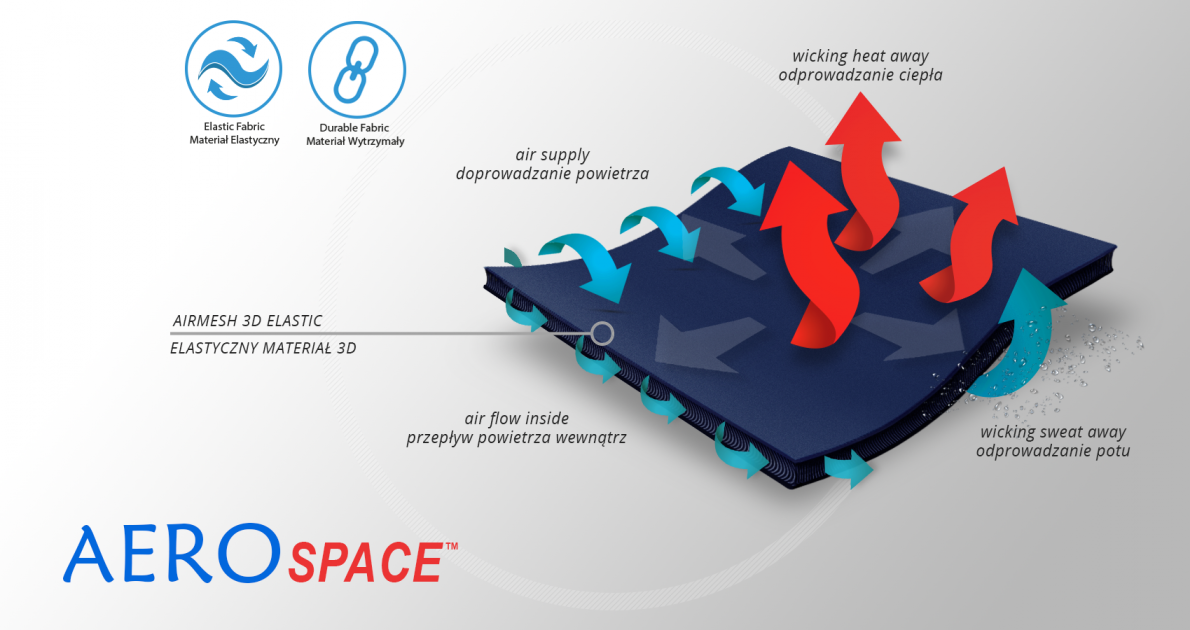
AeroSpace™
AeroSpace™ is an innovative new generation raw material. It is a distance elastic 3D knitted fabric consisting of two layers of facings and an interlacing that creates the appropriate thickness of the raw material and has relieving properties. The knitwear is made of the highest quality polyester yarn - guaranteeing high mechanical strength and spandex ensuring its proper flexibility. This material is characterized by a very low weight, high flexibility and a very large openwork structure, allowing for very easy drainage of sweat from the body and bringing fresh air to the skin. Products made of this raw material are neutral to the secured joint, do not heat or cool it, but ensure its proper compression and fit and reduce muscle vibrations generated during physical exertion. Its thickness and 3D structure perfectly relieves the orthopedic splints, stays or other elements mounted on the product and guarantees velvety softness to the touch.
TECHNOLOGICAL SYSTEMS
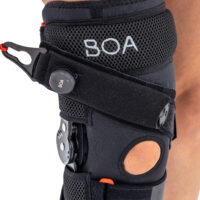
BOA® Fit System
 DIAL IN TO FAST, EFFORTLESS, PRECISION FIT.
Delivering fit solutions purpose-built for performance, the BOA® Fit System is integrated in products across snow sports, cycling, hiking/trekking, golf, running, court sports, workwear, and medical bracing. The BOA® Fit System is engineered with high quality, durable materials that are rigorously field-tested for a micro-adjustable connection that’s built to perform. Each unique configuration is engineered for power without compromising precision in order to deliver a seamless connection between equipment and body. BOA®’s dial and laces are guaranteed for the lifetime of the product on which they are integrated.
DIAL IN TO FAST, EFFORTLESS, PRECISION FIT.
Delivering fit solutions purpose-built for performance, the BOA® Fit System is integrated in products across snow sports, cycling, hiking/trekking, golf, running, court sports, workwear, and medical bracing. The BOA® Fit System is engineered with high quality, durable materials that are rigorously field-tested for a micro-adjustable connection that’s built to perform. Each unique configuration is engineered for power without compromising precision in order to deliver a seamless connection between equipment and body. BOA®’s dial and laces are guaranteed for the lifetime of the product on which they are integrated.
STIFFENINGS
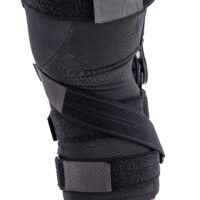
Single axis splint 1RP
Single axis splint 1RP is designed to work in very heavy conditions and allows for transfer loads. This type of splint is primarily used in knee orthoses, where strong lateral stabilization of the knee joint and precise regulation of its mobility are essential. They are used in orthopaedic braces that were designed for heavy patients or for people who use them in extreme conditions. The arms of the splint are made of high-quality hardened steel, hot-coated with a durable powder coating, which makes them very strong and indifferent to the effects of patient’s sweat and salt contained in it. The drop locks are made of very durable and strong acid-resistant steel in which a special hole system with threads were made. The splints are waterproof and can be used in wet and humid environments.
The splint is equipped with the hyperextension knee lock and has following angles of joint flexion: 30 ,50, 70 degrees and the following angles of joint extension: free joint, 20 degrees. The ROM adjustment is made with a special, hardened screws placed into threaded holes marked with a specific angle value we want to lock. In this kind of splint, you can lock not only the angles of flexion and extension of a knee joint, but also the desired range of knee joint movement. Each brace equipped with 1RP splint comes with a free Allen key for these settings. This method of adjusting drop locks in orthopedic splints prevents from their setting by unauthorized persons who do not have a key. The bottom part of the drop lock has an oval shape that allows you to attach a soft anatomic pad made in 3D technology that separates drop lock from the knee. All movable elements of the splints move on galvanized steel rivets and in order to prevent them from seizing, special Teflon sliders were used in these places. Such a construction ensures their maintenance-free operation.
ATTENTION: If the brace is equipped with two side splints, the adjustment should always be made separately on each of the splints, setting the range of motion at the same angle of flexion, extension or lock. Failure to comply with this role will be resulted in the loss of the product warranty.

Setting up
Downloads
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.