OT-23


 Pelvis brace
Pelvis brace CCA
CCA Class I medical device
Class I medical device Compression
Compression Durable
Durable Innovative
Innovative Latex-free
Latex-freeSACROILIAC SI PELVIC HIP BELT WITH PAD AND CCA SYSTEM
Description
SACROILIITIS
Sacroiliac arthritis is a painful condition that affects one or both sacroiliac joints. These joints are located at the junction of the lower spine and pelvis. Sacroiliac arthritis can cause pain and stiffness in the buttocks or lower back and radiating down one or both legs. Usually the pain is exacerbated by prolonged standing or climbing stairs.
Problems from the sacroiliac joints can occur as a result of trauma, rheumatism or a change in spinal biomechanics during pregnancy. Regardless of the cause, treatment includes physiotherapy and physical therapy while stabilising the joints with a professional pelvic belt type OT-23.
Product’s description:
The innovative OT-23 pelvic brace is an advanced medical device designed to stabilise the sacroiliac joints and reduce pain.
The orthosis provides therapeutic compression and relieves pain in the lower back region.
Made from high-quality ActiveSpace™ material, it provides professional compression and allows the product to be customised to the user’s size.
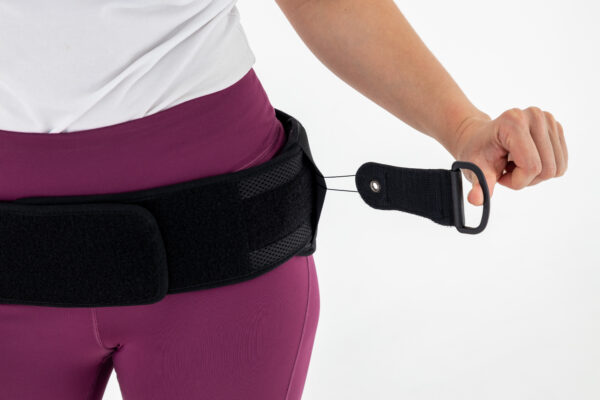
There is compression system CCA and offloading pad in the back of the OT-23 pelvic brace what allowing the proper compression of the product to be set. The system is designed to adjust the brace as precisely as possible to the individual user, thus stabilising the pelvis as effectively as possible. Thanks to this design, it is easier to adjust the product for people with prominent hips or a large abdomen.
Orteza miednicy OT-23 posiada również specjalną pelotę odciążającą, którą możemy umiejscowić w dowolnym miejscu na całej szerokości produktu.
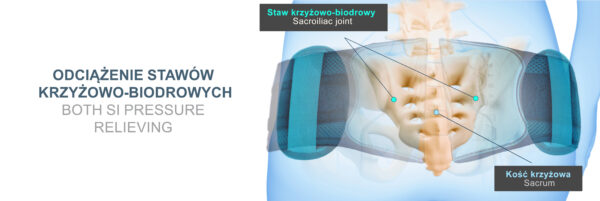
The OT-23 pelvic brace also has a special pressure relieving pad which can be positioned anywhere along the width of the product. Such a solution allows for effective relief of bony prominences, reduction of pressure on the bladder or stabilisation of the sacroiliac joints. Moreover, the pad allows for professional relief of bony prominences in the case of excessive anterior tilt or pelvic rotation.
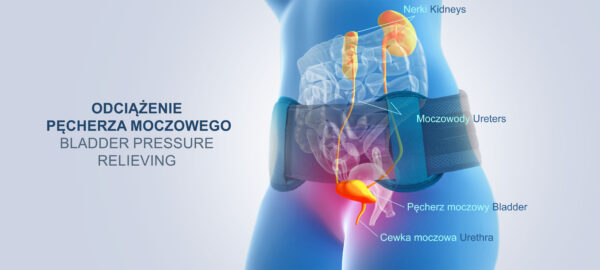
The pelvic brace OT-23 reduces excessive pressure in the sacroiliac joint region and improves pelvic alignment.
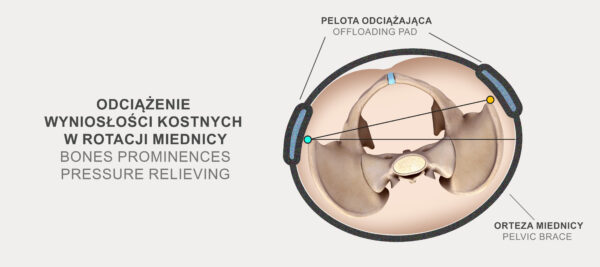
The pelvic brace with pressure relieving pad and CCA compression system supports the lower spine and hip joints, relieving pain and pressure caused by pelvic instability or SI sacroiliac joint dysfunction.
The OT-23 pelvic belt is designed to effectively stabilise and support the pelvis and hips. The product will find use for: back pain, hip pain, pelvic pain, pubic conjunctival dysfunction, pelvic rim pain, pear-shaped syndrome or excessive anterior pelvic tilt.
Purpose of use:
- Sacroiliac arthrosis
- Sacroiliac Joint Syndrome.
- Sacroiliac joint instability
- Pubic symphysis dehiscence
- Sacroiliac joint dysfunction
- Painful syndrome of the pelvis muscles or tendinopathy
- Lumbo-sacral spine spondylosyndesis
- Sacral stress fracture
Sizes
| Size | Hip circumference at the widest point | How to measure |
|---|---|---|
| M | 88 – 100 cm | 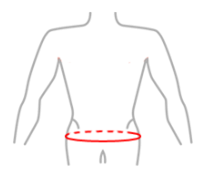 |
| L | 100 – 112 cm | |
| XL | 112 – 125 cm |
Gallery
Technology
MATERIALS
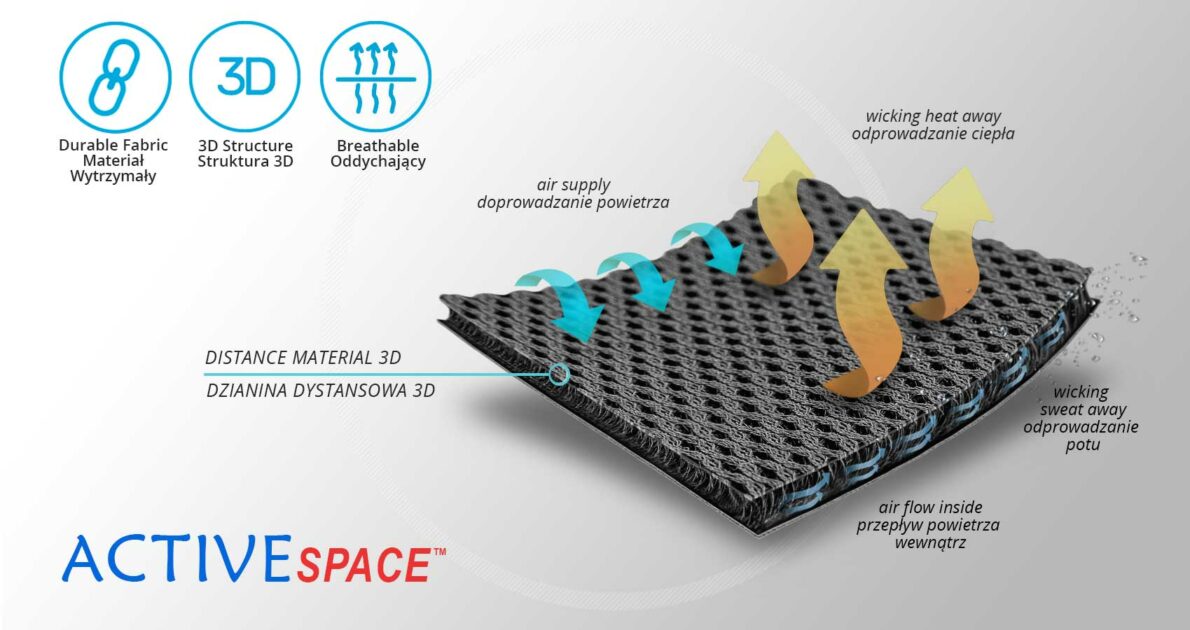
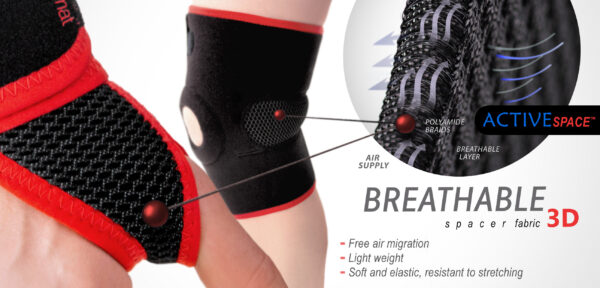
ActiveSpace™
ActiveSpace™ is a spacer, polyamide 3D lamination with high skin ventilation. It is very lightweight, consisted of 2 layers. Between them, we use polyamide braids. ActiveSpace™ is not elastic what improves stabilization. Inside the lamination, between 2 layers, the air flows freely, maintaining minimal water and moisture absorption. Waterproof material.
MaxComfort™
MaxComfort™ is a new generation of multilayer material with excellent relief and functionality. The use of a product made of this material is a perfect stabilization of the limb and very high comfort of use.
From the side of the body, there is a soft, skin-friendly knitted fabric with OekoTex Standard100 certificate. The next layer is a soft polyurethane foam, which ensures the comfort of using the orthosis, fill the space between the orthosis and the limb, increases the contact surface of the orthosis to the patient's skin, which reduces local pressure on the body and has anti-bedsore properties. The next layer of the material is technical polyethylene foam, whose task is to relieve the structural elements of the orthosis from the patient's body. The last layer of this material is a technical fabric with a self-adhesive function that allows you to attach various types of circumferential bands and other details necessary for the proper functioning of the orthosis.
TECHNOLOGICAL SYSTEMS
CCA Compression System

Dial CCA Compression System was designed to use it in the snowboard shoes, because ordinary shoelace was too weak in extreme using. Typical shoestring sprang and snowboard shoes wasn’t casing construction what could allow using the steel fastening.
Steel wire and polyamide grommets allow to use steel fastening. Later, this system was used in other sport shoes such as: professional shoes for cycling or running.
In orthopaedic field the compression system was adapted early and it’s used in e.g. ankle, wrist or back braces. The system is characterized by firm fastening that is non-elastic, easy and secure. There is impossible to loose (only in case of damage) it what guarantees the best stabilization.
Our CCA System provides different levels of compression. It’s based on differential polyamide grommets , steel wires covered by plastic and knobs. The additional equipment is the special element that is easy to attach what allows to modify device’s shape or circumference.
The CCA System is the compression solution so you can use it only after putting the brace on the body.
STIFFENINGS
Hip supports
Hip supports are made of ABS material. Their purpose is to effectively relieve the compression straps in the pelvic brace. Thanks to them, the high impact force of the belts on the patient's body is distributed over a much larger area, so that the local pressure on the body is much smaller. The pads have special cutouts and guides for the compression belts. On one of the orthoses, there is also a cut-out which is the base for the toothed belt.

Setting up
Downloads
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.