Pelvic stabilization belt AM-PCS-02


PELVIC BELT
Description
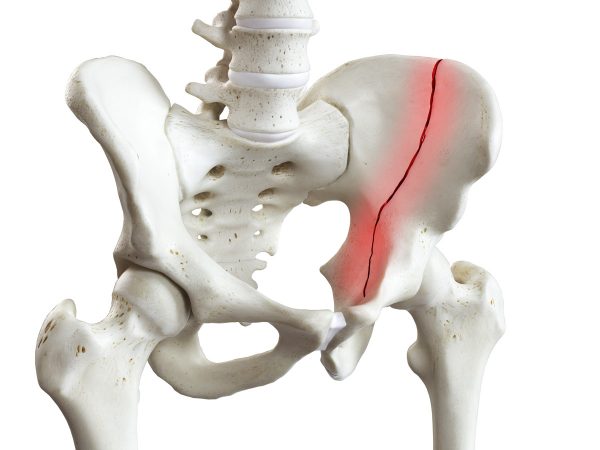
Pelvic fracture
Fractures of the pelvis are uncommon but really serious. Because the pelvis is in proximity to major blood vessels and organs, pelvic fractures may cause extensive bleeding and other injuries that require urgent medical treatment. The pelvis consists of two hip bones, sacrum and tail bone (coccyx). Pelvic fractures can be described as “stable” or “unstable”, which is associated with side rotation. Stable pelvic fractures usually do not require surgery. In this case, pelvic immobilization by professional pelvis belt AM-PCS-02 should be sufficient.
Product’s description
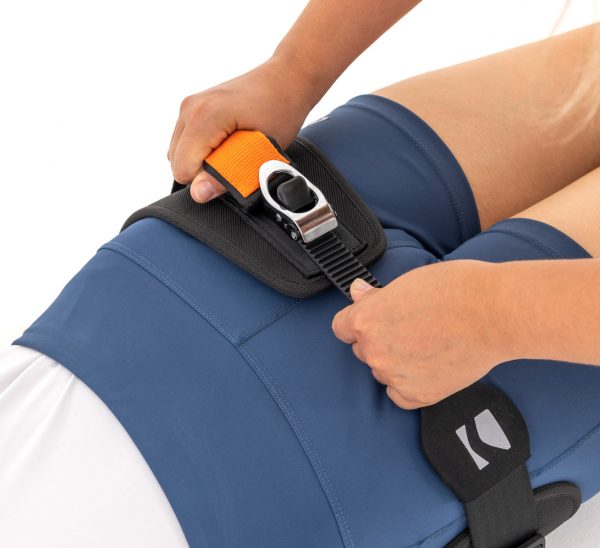
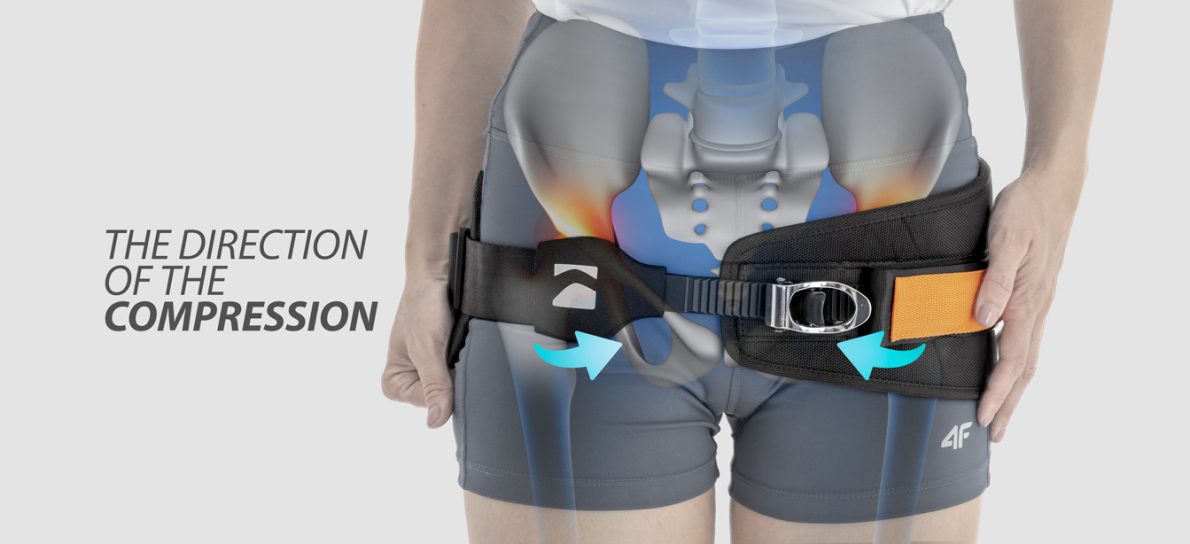
Pelvic belt AM-PCS-02 is a professional medical sling for pelvic immobilization. The belt consists of offloading belt, compression strap and advanced compression system CALIPER BUCKLE™.
Anatomic shaped offloading belt is made of high quality fabric PowerLift™.
The offloading sling is anatomic shaped and a slightly elastic offering firm pelvic immobilization without limited hips range of motion.
What is more, the belt does not cover the abdominal part so changing dressings and drains is simple, quick and does not require removal of the product.
On the offloading sling there is a non-elastic, firm, made of nylon strap with innovative self-locking clamp. Non-elastic strap makes our pelvic sling AM-PCS-02 firm and perfect for pelvic immobilization. It offers high quality compression, necessary for correct pelvic stabilization.
AM-PCS-02 pelvic belt is fastened with innovative compression system CALIPER BUCKLE™.
CALIPER BUCKLE™ is a firm system offering safe and effective pelvic stabilization.
Our pelvic belt AM-PCS-02 can be used in case of stable and unstable pelvic fractures (according to doctor’s recommendations). The belt maintains constant compression, improves healing and prevents displacement of fractures.
Our pelvic sling AM-PCS-02 offers pelvic immobilization and can be used at home and medical and rehabilitation centers.
BENEFITS
- lightweight, brreathable material
- self-locking compression system CALIPER BUCKLE™
- anatomic shape
- free from pressure on the abdomen
- free from pressure on veins and arteries
- effective stabilization
Przeznaczenie
– złamanie kości miednicy
– konieczność unieruchomienia miednicy
Sizes
| Size | Hips circumference | How to measure |
|---|---|---|
| M | 80 cm – 105 cm | 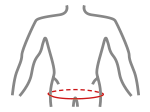 |
| L | 105 cm – 140 cm |
Fits for both hips.
Gallery
Technology
MATERIALS
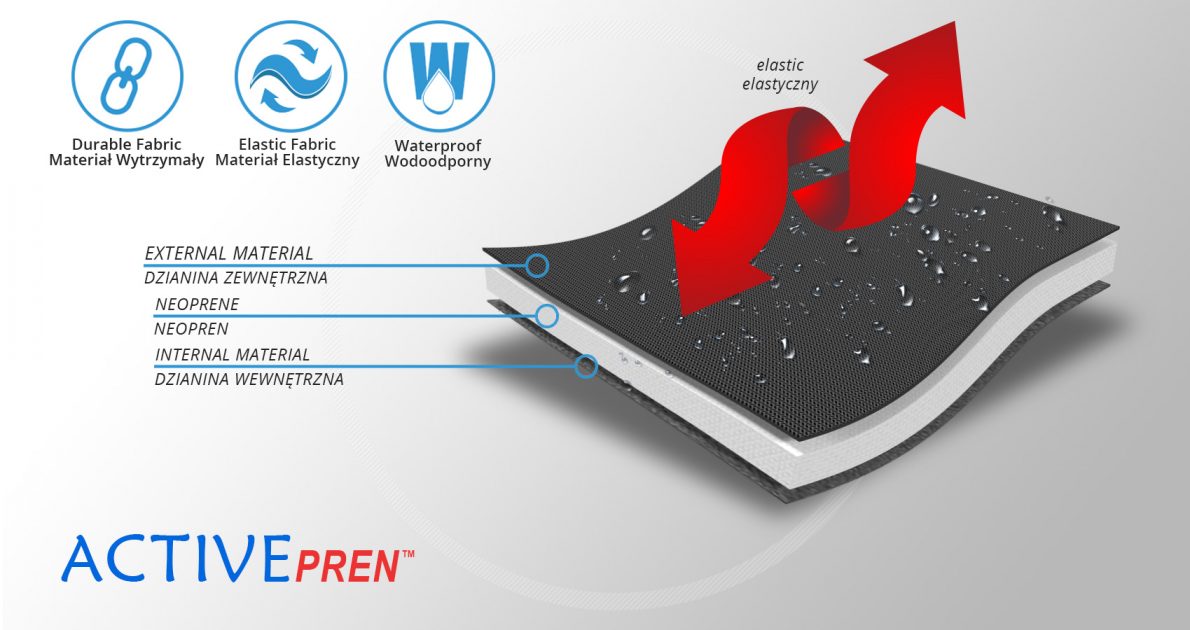
ActivePren™
ActivePren™ is an active three-layer material consisting of two elastic jersey cover fabrics and a core made of neoprene foam. This material is characterized by softness and high flexibility. A very important advantage of this material is the fact that it is not a knitted product, it does not have thick fibers, so that the weaves of the material do not imprint on the patient's skin and do not cause abrasionsin places of high compression. Products made of ActivePren are the strongest and most effective stabilizing orthoses available on the market.
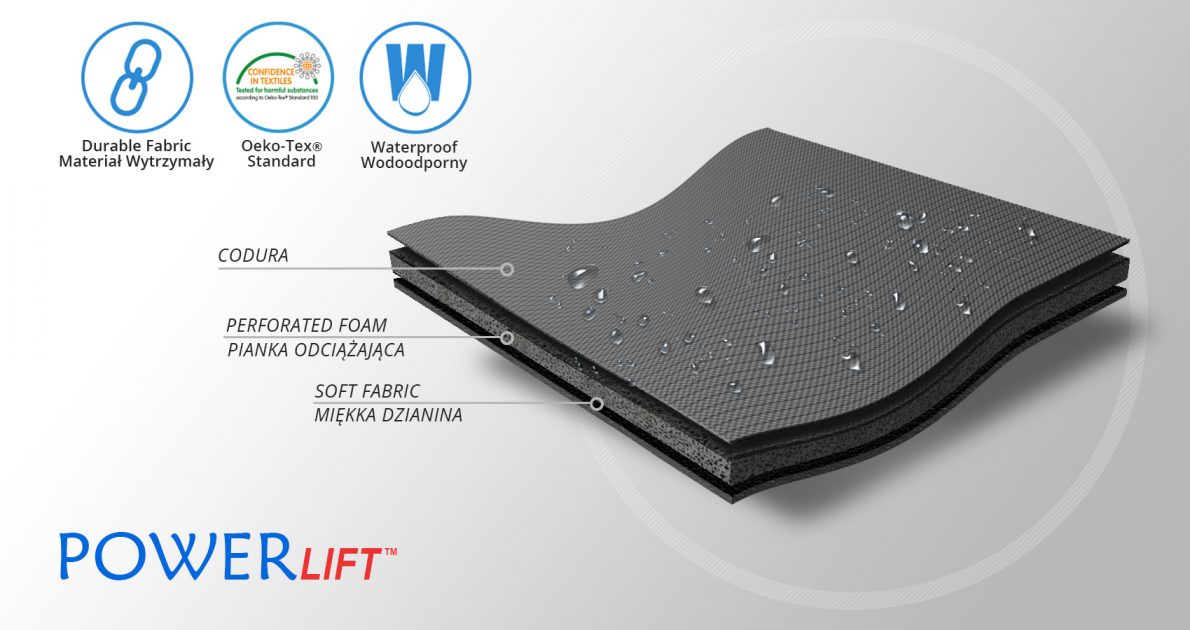
PowerLift™
PowerLift™ is an off-loading raw material with a three-layer structure. It consists of a very strong outer fabric, highly abrasion-resistant made of polyamide with a polyurethane coating with Teflon finish, relieving EVA foam and a soft cover knit. Waterproof material.
TECHNOLOGICAL SYSTEMS
CALIPER BUCKLE

CALIPER BUCKLE– it’s the special very efficient system of closure. The system consist of elastic ladder strap and metal caliper buckle with a clever ratcheting system. This system is very easy to use and there is enough to raise the cocking lever. The caliper buckle system is used for producing the orthopedic devices, required precise and strong compression. This system of closure is equipped with release lever that turns the compression immediately off. This fast ‘compression-release’ function is very useful in case of too strong compression, resulting in breathing problems of patient.
Setting up
Downloads
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.