Wrist stabilization EB-N-01 BLACK MELANGE


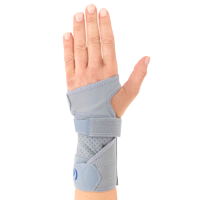
 Wrist brace
Wrist brace Anatomic wrist splint
Anatomic wrist splint Cast replacement
Cast replacement Class I medical device
Class I medical device Compression
Compression Durable
Durable ER
ER Innovative
Innovative Skin-friendly
Skin-friendlyWRIST BRACE
Description
Wrist support made of ProFit™.
Parts are made of innovative material called AirSanmed™.
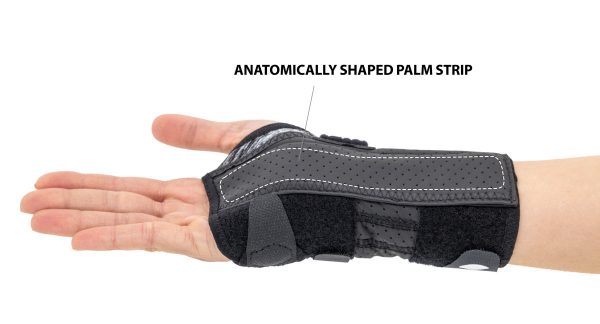
The Wrist Support is equipped with anatomically shaped, malleable and removable strip on palm. The fastening VELCRO tape allows the perfect fit to the wrist.
Purpose of use
- after wrist injuries
- bursitis
- joint degeneration or inflammation
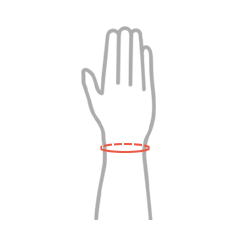
Sizes
| Size | Wrist circumference | How to measure |
| S | 13-15 cm (5,1″-5,9″) |
 |
| M | 15-17 cm (6,1″-6,7″) |
|
| L | 17-19 cm (6,9″-7,5″) |
|
| XL | 19-21 cm (7,7″-8,3″) |
Total length of product: 17 cm (6,7″)
Left/right side available.
Colors
Gallery
Technology
MATERIALS
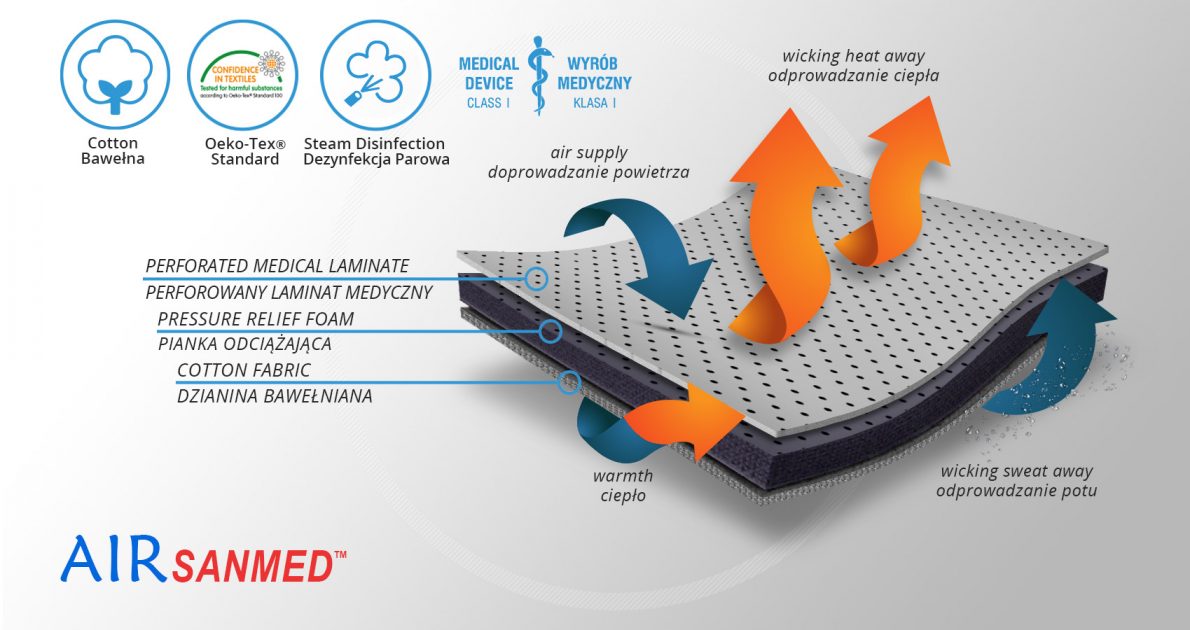
AirSanmed™
AirSanmed™ isn’t elastic what provides excellent stabilization. The skin has contact with cotton terry with Oeko-Tex Standard 100 certificate. There is semi-rigid perforated foam EVA inside that protect the skin against the metal splints influence. External side of the fabric is perforated medical laminate with antibacterial properties of Silver Zeolite. It provides long-term efficacy and prevents the most dangerous infectious microorganisms such as MRSA and E.coli. AirSanmed™ is in accordance with Health Minister`s ordinance of 3 November 2004 and Council Directive 93/42/EWG of 14 June 1993.
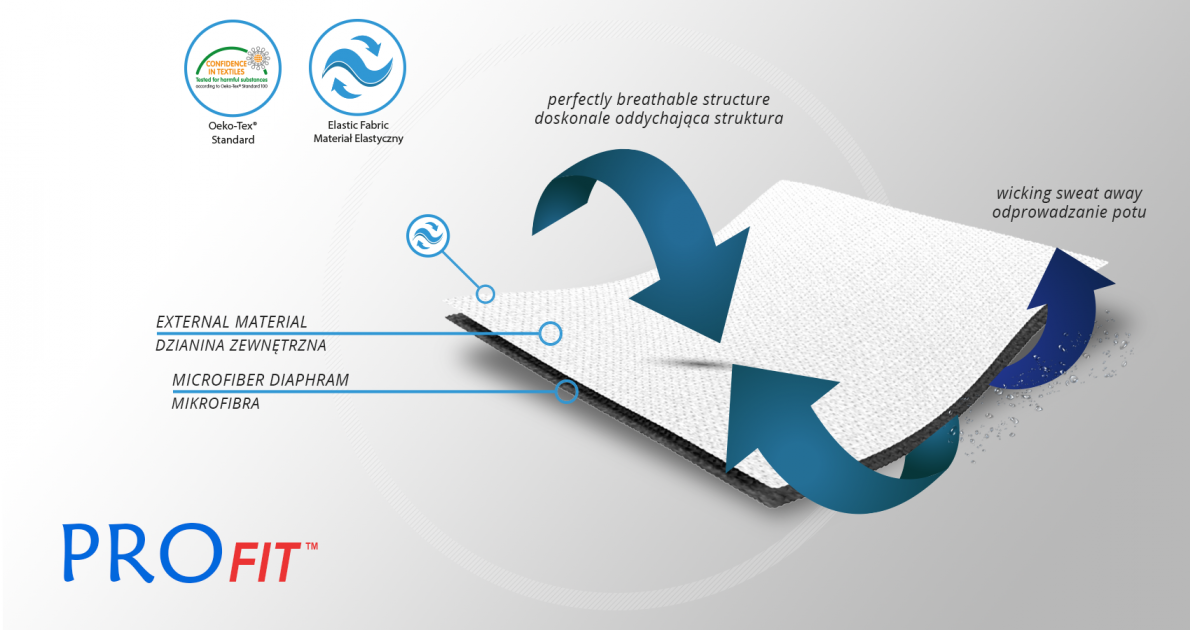
ProFit™
ProFit™ is a double-layer fabric that is elastic in all directions and fits to limb precisely. The external side is made of spandex providing excellent compression and absorbs muscle shocks in physical activity. The internal side consists of microfibre, cotton and elastane what makes it skin-friendly, breathable and provides velvet softness. ProFit™ is friendly and neutral for the skin and has Oeko-Tex Standard 100 certificate. In some products this material can have an internal elastic cotton layer.
STIFFENINGS
Palm strip
Setting up
Downloads
Accessories
ACCESSORIES / PRODUCTS TO BE USED WITH
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.