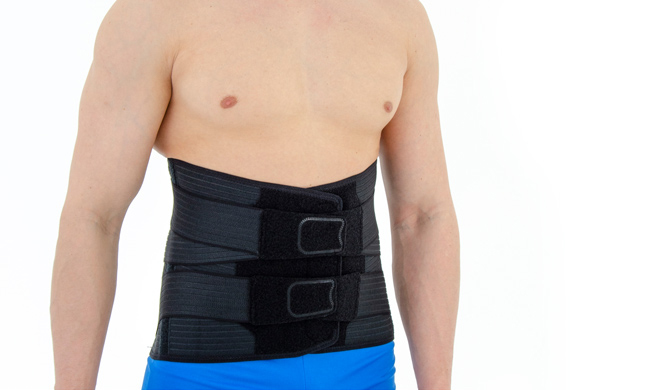
Back brace OT-10 BLACK

 TLSO
TLSO Breathable
Breathable Class I medical device
Class I medical device Machine washable
Machine washable Recommended by specialists
Recommended by specialists Skin-friendly
Skin-friendlyBACK BRACE WITH X-STRAPS
Description
Spinal stenosis
Spinal stenosisis a condition in which spinal canal starts to narrow. It’s mostly in adults 40 or more and the main reason is arthritis. However, spinal stenosis may be result of herniated discs, injuries, tumors and Paget’s disease. Mostly conditions, leading to spinal stenosis, are caused by stressed repetitive activities during day. For this reason, physical workers and employees with sedentary lifestyle suffer from spinal stenosis commonly.
Spine is made up of a series of vertebrae and shock-absorbing discs. Such structure protects spinal cord, connecting brain to the body, against the injuries. When the spinal canal becomes more narrow, the open space between vertebrae is smaller, and there is less place.
At that time, the tightness can pinch the spinal cord or the nerves around it, causing pain, tingling, or numbness in your legs, arms, or torso. Furthermore, spinal stenosis may result in sciatica, foot drop, walking difficulties and loss of bladder or bowel control!
Together with traditional pharmacological therapy, you should use our active back brace OT-10.
Product’s description
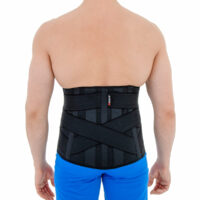
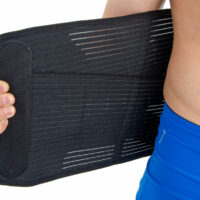
Our back brace OT-10 is an excellent product, used for lower back support. The brace is made of innovative material ActivRubber II™ available in black and beige colors.
It is made of innovative fabric ActivRubber II™. Available in beige or black color.
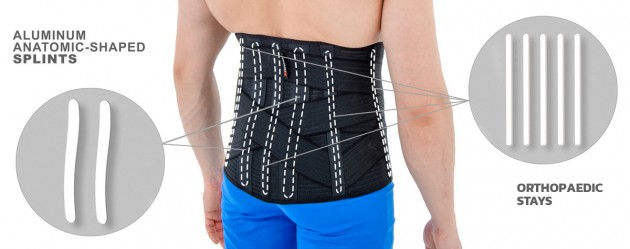
Our OT-10 back brace is equipped with rear aluminum anatomic-shaped splints and orthopaedic stays which support muscles, ligaments and vertebrae.
Lower back brace OT-10 has special rear X-straps. This solution provides perfect fitting. The anatomic splints reflects the your lordosis with your every motion. The splints support vertebrae and soft tissues such as ligaments and muscles. X-straps and splints prevent against lower back injuries and relieve the pain associated with overused conditions.
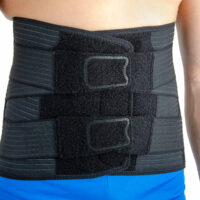
Wide, front Velcro closures provides extra support for abdominal wall and wide range of circumferential adjustment.
Use our back brace OT-10 brace in daily activities: in work, home or during walking. The brace supports your spine and prevents against the tissue’s injuries. This product relieves the pain and allows to live normally.
Purpose of use
– Pre- and Post-Surgical Stabilization
– Degenerative Spinal pathologies
– Disc Hernia
– Spondylolithesis
– Spondylolysis
– Acute Back Pain
– Spinal Instability
– Rehabilitation and Prevention
– Herniated disc / discopathy
– Sciatica
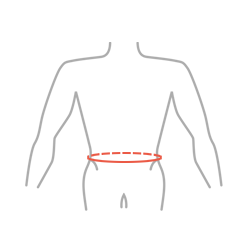
Sizes
| Size | Waist circumference | How to measure |
| S | 65-75 cm (25,6″-29,5″) |
 |
| M | 75-85 cm (29,5″-33,5″) |
|
| L | 85-97 cm (33,5″-38,2″) |
|
| XL | 97-110 cm (38,2″-43,3″) |
|
| 2XL | 110-125 cm (43,3″-49,2″) |
|
| 3XL | 125-145 cm (49,2″-57,1″) |
Total height of the product:
Front: 24 cm (9,4″)
Back: 33 cm (13″)
Colors
Gallery
Technology
MATERIALS
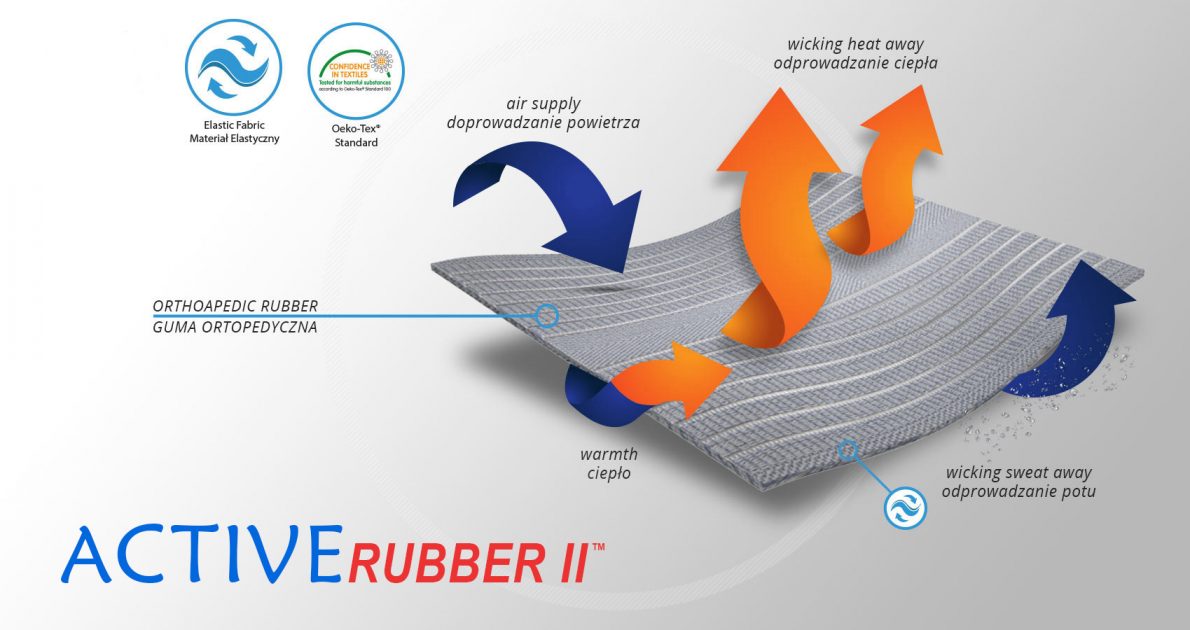
ActiveRubber II™
ActiveRubber II™ is strong and bidirectional elasticity. It can be stretched, increasing the length and width, what improves compression. Between the braids air flows freely and skin can breathe. In addition in high compression, these rubber braids provide a gentle massage for skin. Our orthopedic rubber is very friendly for skin.
STIFFENINGS
Orthopedic profiled stays
Orthopedic stays are made of special spring steel, covered with a plastic coating and have rounded and protected ends. They can come in various widths and thicknesses as well as different hardness. They can be pre-shaped or flat. They work in one direction and adapt to the shape of the body while stabilizing the laterally protected part of the body. They are perfectly protected against corrosion, so they are resistant to water, moisture and sweat. Products equipped with them can be washed without removing them from the orthosis. They adjust to the body. The orthopedic stays cannot bend and that is why, they cannot correct the body posture or the secured joint.

Orthopedic stays from ABS
Orthopedic stays available in various width, thickness and shapes are cut from ABS boards. Their role is not to stiffen the orthosis in order to support a joint or other part of the body, but to prevent wrinkling and uncontrolled movement of the fabrics of the orthosis./p>

Plastic stays
They come in various widths and thicknesses, are made of various types of plastics, such as polyamide, ABS or acrylic, and these features determine their stiffness. Thanks to their design, they are resistant to water, moisture and sweat. Products equipped with them can be washed without having to remove them from the orthosis. Our plastic stays work only in one direction, perfectly stabilize the laterally protected part of the body, adjusting to it at the same time and have a memory function, thanks to which they always return to their original shape. This function causes the stays in the orthosis to stabilize the swollen limb immediately after the injury and after the swelling has come off. The plastic stays cannot bend and that is why, they cannot correct the body posture or the secured joint.
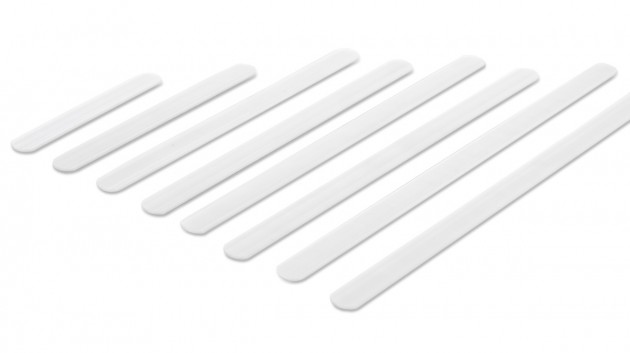
Profiled aluminum stays
These are splints and orthopedic stays of various thickness and width, which are made of various types of aluminum alloys. All these splints and stays, before mounting to a given orthosis, have been pre-profiled, which allows for fitting the product to the body of a specific patient. However, for the correct operation of the device, they should be precisely bent to the patient's body by an orthopedist, physiotherapist or orthopedist technician. Only this action guarantees the proper protection and support of the patient's body.

Stiffeners of thin ABS
Stiffeners made of a thin ABS plate are very light and protect the correct shape of the brace, but do not stiffen individual parts of the patient's body. The brace has the correct circumferential shape, but does not have the compensating and stiffening function of the protected joints and other protected parts of the body.

Setting up
Downloads
Accessories
ACCESSORIES / PRODUCTS TO BE USED WITH
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.